
Tom Johnson (University Hospitals Bristol & Weston NHS Foundation Trust, Bristol, UK) outlines how the arrival of intravascular lithotripsy (IVL) has moved the dial for coronary calcium treatment within his centre in the last five years.
Calcium is every interventionist’s foe. Sadly, its independent association with advancing age, hypertension, and ST-elevation myocardial infarction (STEMI) presentation1 increases our likelihood of encountering significant calcific disease during unplanned, emergency percutaneous coronary intervention (PCI). Consequently, devices to overcome calcium are needed in every interventionist’s toolbox.
Shockwave IVL disrupted the landscape of UK interventional cardiology early in 2018 with a live-case undertaken by Keith Oldroyd at Golden Jubilee, Glasgow. Undoubtedly, the arrival of Shockwave IVL provided renewed enthusiasm for the management of complex calcific coronary disease. Simplicity of use has resulted in IVL becoming the ‘go-to’ adjunctive tool for calcium modification. However, associated costs have required a thoughtful approach to technology adoption.
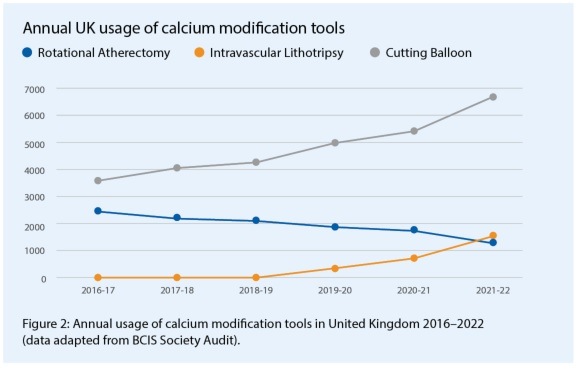
 IVL arrived in Bristol in July 2018 and through our interest in intracoronary imaging we elected to combine IVL adoption with a more thorough assessment of coronary calcification. It has been well demonstrated that intravascular ultrasound (IVUS) and optical coherence tomography (OCT) reveal significantly more calcium than appreciated by coronary angiography alone.2 Furthermore, we now have quantitative thresholds that predict stent under-expansion and facilitate tailored decision making for adjunctive calcium modification.3 Consequently, our adoption of intracoronary imaging to guide the management of calcific coronary disease resulted in a 60.6% increase in modification with either rotational atherectomy (RA) or IVL, and a more than doubling of intracoronary imaging guidance in severe calcium cases (see Figure 1). Looking at the national adoption of IVL, using data available from the annual British Cardiovascular Intervention Society (BCIS) audit results, there has been a steady rise in IVL use, with a reduction in RA, similar to our experience, but additionally an impressive rise in the use of cutting balloon technologies, corresponding with the arrival of IVL (Figure 2).
IVL arrived in Bristol in July 2018 and through our interest in intracoronary imaging we elected to combine IVL adoption with a more thorough assessment of coronary calcification. It has been well demonstrated that intravascular ultrasound (IVUS) and optical coherence tomography (OCT) reveal significantly more calcium than appreciated by coronary angiography alone.2 Furthermore, we now have quantitative thresholds that predict stent under-expansion and facilitate tailored decision making for adjunctive calcium modification.3 Consequently, our adoption of intracoronary imaging to guide the management of calcific coronary disease resulted in a 60.6% increase in modification with either rotational atherectomy (RA) or IVL, and a more than doubling of intracoronary imaging guidance in severe calcium cases (see Figure 1). Looking at the national adoption of IVL, using data available from the annual British Cardiovascular Intervention Society (BCIS) audit results, there has been a steady rise in IVL use, with a reduction in RA, similar to our experience, but additionally an impressive rise in the use of cutting balloon technologies, corresponding with the arrival of IVL (Figure 2).
 It is important to reflect that our real-world use of IVL differs significantly from the cohorts recruited to the DISRUPT-CAD series of studies,4‒6 where amongst others, recent acute coronary syndrome (ACS) presentation, left main stem (LMS) disease, chronic total occlusion (CTO), and significant left ventricular dysfunction (ejection fraction [EF] <40%) all were excluded.
It is important to reflect that our real-world use of IVL differs significantly from the cohorts recruited to the DISRUPT-CAD series of studies,4‒6 where amongst others, recent acute coronary syndrome (ACS) presentation, left main stem (LMS) disease, chronic total occlusion (CTO), and significant left ventricular dysfunction (ejection fraction [EF] <40%) all were excluded.
As acknowledged earlier, significant calcium associates with STEMI, and consequently a device that is easy to use provides a novel solution during emergency intervention, 38.8% of our cases have been in the setting of ACS, similar to the experience of another high-volume UK centre.7 Additionally, the excellent safety profile demonstrated in the DISRUPT-CAD series reassured us in extending the use of the device in higher risk patient/lesion subsets; in our first two years of IVL treatment, 9.2% of cases were unprotected LMS and 8.2% were CTO. With increasing lesion complexity and burden of disease, we have observed the need for extensive IVL treatment with 30% of cases utilising all 80 pulses and 5% of cases requiring multiple balloons. Consequently, the arrival of Shockwave C2+, providing 50% more shocks (120 pulses vs. 80 pulses), offers an advantage in cases of the highest burden calcification.
Our adoption of intracoronary imaging in calcific disease has led to an awareness of differing patterns of calcium and consequently generated significant debate regarding the optimal tools to achieve effective modification, thereby facilitating a durable stent result. Beyond quantifying high-burden disease including >180-degree arc, thickness >500m and longitudinal extent >5mm3, the location and qualitative nature of calcium influences our choice of modification device.
 Intracoronary imaging and post-mortem analysis of calcified coronary arteries have extended our knowledge of the characteristics of calcific plaques, with calcified sheet
Intracoronary imaging and post-mortem analysis of calcified coronary arteries have extended our knowledge of the characteristics of calcific plaques, with calcified sheet
being the most commonly encountered but eruptive calcified nodule causing the greatest concern through association with plaque instability, coronary thrombosis and sudden cardiac death.8,9
Interestingly, calcified nodules have been observed most frequently in the proximal and mid portions of the major epicardial vessels and their eruptive nature has been attributed to highly mobile vessel segments, where the calcium is exposed to greater torsional stress.8 Regardless of eruptive potential, calcified nodules defined by IVUS have been associated with poorer clinical outcomes10 and consequently greater attention to their modification and treatment is needed. Recently, a pooled analysis of cases recruited to the DISRUPT-CAD study series4‒6,11 has facilitated an assessment of IVL’s impact on calcified nodules.12 This small OCT cohort analysis, with or without calcified nodule, highlighted an association of calcified nodules with the right coronary artery (51.9%—promoting the connection with vessel mobility), higher burden calcium, and requirement for greater predilatation (48.1% vs. 27.3%, p=0.005).
Despite greater calcium burden, IVL treatment of calcified nodules achieved comparable stent expansion (mean 104.9% vs. 99.4% for non-calcified nodule lesions, p=0.87) and minimal stent areas (6.3mm2 vs. 6.0mm2, p=0.41). Interestingly all eruptive nodules were deformable, whereas just over one third of non-eruptive nodules were non-deformable with persisting stent eccentricity/asymmetry. Larger-scale studies are needed and at present, consensus has not been achieved with regard to device selection for differing patterns of calcium, with multiple algorithms available to consider.13,14
Selecting between ablative and balloon-based technologies is left to the discretion of the operator, but the ease of use and excellent safety profile of IVL has resulted in Shockwave’s use for all but uncrossable lesions.
References
1. Genereux P, Madhavan MV, Mintz GS, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J Am Coll Cardiol. 2014;63:1845‒1854. doi: 10.1016/j.jacc.2014.01.034
2. Wang X, Matsumura M, Mintz GS et al. In Vivo Calcium Detection by Comparing Optical Coherence Tomography, Intravascular Ultrasound, and Angiography. JACC Cardiovascular imaging. 2017;10:869‒879. doi: 10.1016/j.jcmg.2017.05.014
3. Fujino A, Mintz GS, Matsumura M et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182-e2189. doi: 10.4244/EIJ-D-17-00962
4. Hill JM, Kereiakes DJ, Shlofmitz RA et al. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. J Am Coll Cardiol. 2020;76:2635‒2646. doi: 10.1016/j.jacc.2020.09.603
5. Ali ZA, Nef H, Escaned J et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Stenoses: The Disrupt CAD II Study. Circ Cardiovasc Interv. 2019;12:e008434. doi: 10.1161/CIRCINTERVENTIONS.119.008434
6. Brinton TJ, Ali ZA, Hill JM et al. Feasibility of Shockwave Coronary Intravascular Lithotripsy for the Treatment of Calcified Coronary Stenoses. Circulation. 2019;139:834-836. doi: 10.1161/CIRCULATIONAHA.118.036531
7. Yeoh J, Kanyal R, Pareek N, et al. Intravascular lithotripsy in the treatment of coronary artery calcification in a high-risk real world population. Catheter Cardiovasc Interv. 2023. doi: 10.1002/ccd.30546
8. Torii S, Sato Y, Otsuka F, et al. Eruptive Calcified Nodules as a Potential Mechanism of Acute Coronary Thrombosis and Sudden Death. J Am Coll Cardiol. 2021;77:1599‒1611. doi: 10.1016/j.jacc.2021.02.016
9. Sugiyama T, Yamamoto E, Fracassi F, et al. Calcified Plaques in Patients With Acute Coronary Syndromes. JACC Cardiovasc Interv. 2019;12:531-540. doi: 10.1016/j.jcin.2018.12.013
10. Morofuji T, Kuramitsu S, Shinozaki T, et al. Clinical impact of calcified nodule in patients with heavily calcified lesions requiring rotational atherectomy. Catheter Cardiovasc Interv. 2021;97:10‒19. doi: 10.1002/ccd.28896
11. Saito S, Yamazaki S, Takahashi A, et al, Disrupt CADIVI. Intravascular Lithotripsy for Vessel Preparation in Severely Calcified Coronary Arteries Prior to Stent Placement – Primary Outcomes From the Japanese Disrupt CAD IV Study. Circulation Journal: Official journal of the Japanese Circulation Society. 2021;85:826‒833. doi: 10.1253/circj.CJ-20-1174
12. Ali ZA, Kereiakes D, Hill J, et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Calcified Nodules. JACC Cardiovasc Interv. 2023. doi: 10.1016/j.jcin.2023.02.015
13. De Maria GL, Scarsini R, Banning AP. Management of Calcific Coronary Artery Lesions: Is it Time to Change Our Interventional Therapeutic Approach? JACC Cardiovasc Interv. 2019;12:1465‒1478. doi: 10.1016/j.jcin.2019.03.038
14. Fan LM, Tong D, Mintz GS, et al. Breaking the deadlock of calcified coronary artery lesions: A contemporary review. Catheter Cardiovasc Interv. 2021;97:108‒120. doi: 10.1002/ccd.29221











