Medtronic has announced new, long-term data from the SPYRAL HTN-ON MED clinical trial that showed subjects who underwent radiofrequency renal denervation with the Symplicity Spyral renal denervation system had significantly greater reductions in 24-hr ambulatory systolic blood pressure, and office-based systolic blood pressure compared to sham patients at two years.
The data were presented at TCT 2024 (27–30 October, Washington, DC, USA) by David Kandzari (Piedmont Heart Institute, Atlanta, USA), principal investigator in the SPYRAL HTN-ON MED trial.
“These findings are a key step toward informing the medical community of the long-term effectiveness with radiofrequency renal denervation as a treatment for uncontrolled hypertension,” Kandzari was quoted as saying in a press release issued by Medtronic. “Importantly, at two years, we continue to see Symplicity is safe and consistent with clinically meaningful and significant blood pressure reductions. These data further substantiate sustained blood pressure reductions consistently observed in across the SPYRAL and Global Symplicity clinical programmes.”
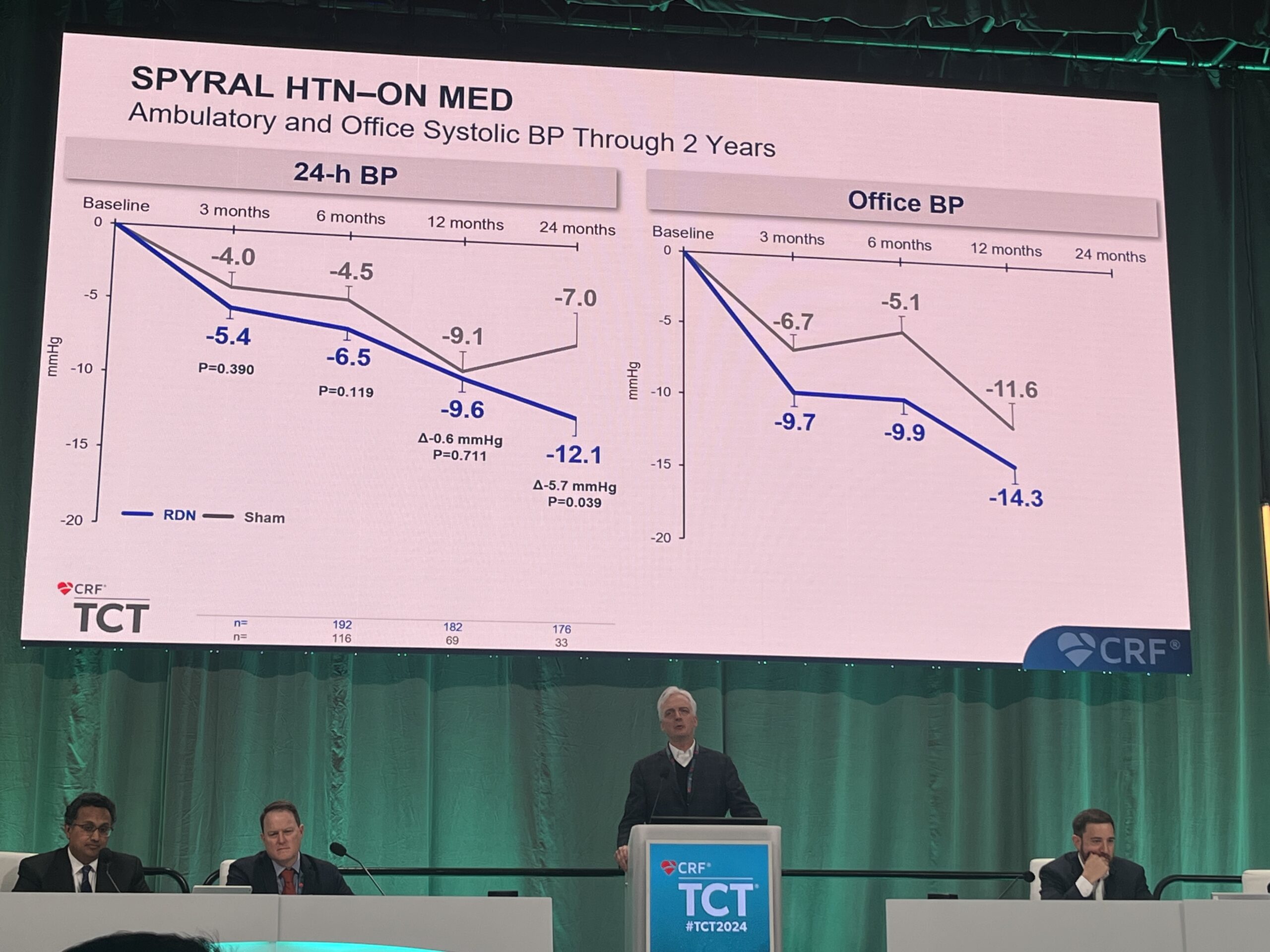
At two years, the data showed significant group differences in 24-hr ambulatory systolic blood pressure and office-based systolic blood pressure in favour of renal denervation, despite significantly more medications detected in the sham group.
At 24 months ambulatory systolic blood pressure reduced by -12.1 mmHg in RDN group vs. -7mmHg in sham group (treatment difference: -5.7 mmHg; p=0.039), and office-based systolic blood pressure: -17.4 mmHg in the RDN group vs. -9.0 mmHg in the sham group (treatment difference: -8.7 mmHg; p=0.0034). Long-term safety with no confirmed renal artery stenosis greater than 70% in the Spyral group at two years
Medtronic intends to investigate multi-organ (hepatic artery and renal artery) denervation with the Symplicity Spyral catheter. The planned Global Pilot study, SPYRAL GEMINI, will investigate the safety and efficacy of the multi-organ ablation approach in uncontrolled hypertension patients who are both on and off medications.
The utilisation of Symplicity Spyral in the hepatic artery is investigational and not approved for use.
The company is also expanding the GSR-DEFINE clinical trial to sites in the USA. The GSR-DEFINE trial is an extension of the Global SYMPLICITY registry, and is a prospective, all-comer observational study in 251 sites across 55 countries, including 3,000 patients from the GSR study and enrolling up to an additional 2,000 patients globally.
Approved for commercial use in over 75 countries around the world, the Symplicity Spyral renal denervation system is currently limited to investigational use in Japan.