The Evoque (Edwards Lifesciences) tricuspid valve replacement system demonstrated superiority compared to medical therapy alone for the one-year primary endpoint of the TRISCEND II trial.
TRISCEND II is a randomised controlled pivotal trial designed to study the Evoque system with optimal medical therapy (OMT) compared to OMT alone with 2:1 randomisation.
The data, presented at the 2024 TCT meeting (27–30 October, Washington, DC, USA), by Susheel Kodali (Columbia University Irving Medical Center, New York, USA) and Suzanne Arnold (University of Missouri-Kansas City, Kansas City, USA) included the full cohort of 400 patients. One-year primary endpoint outcomes have been simultaneously published in The New England Journal of Medicine, and one-year quality-of-life (QoL) outcomes in the Journal of the American College of Cardiology.
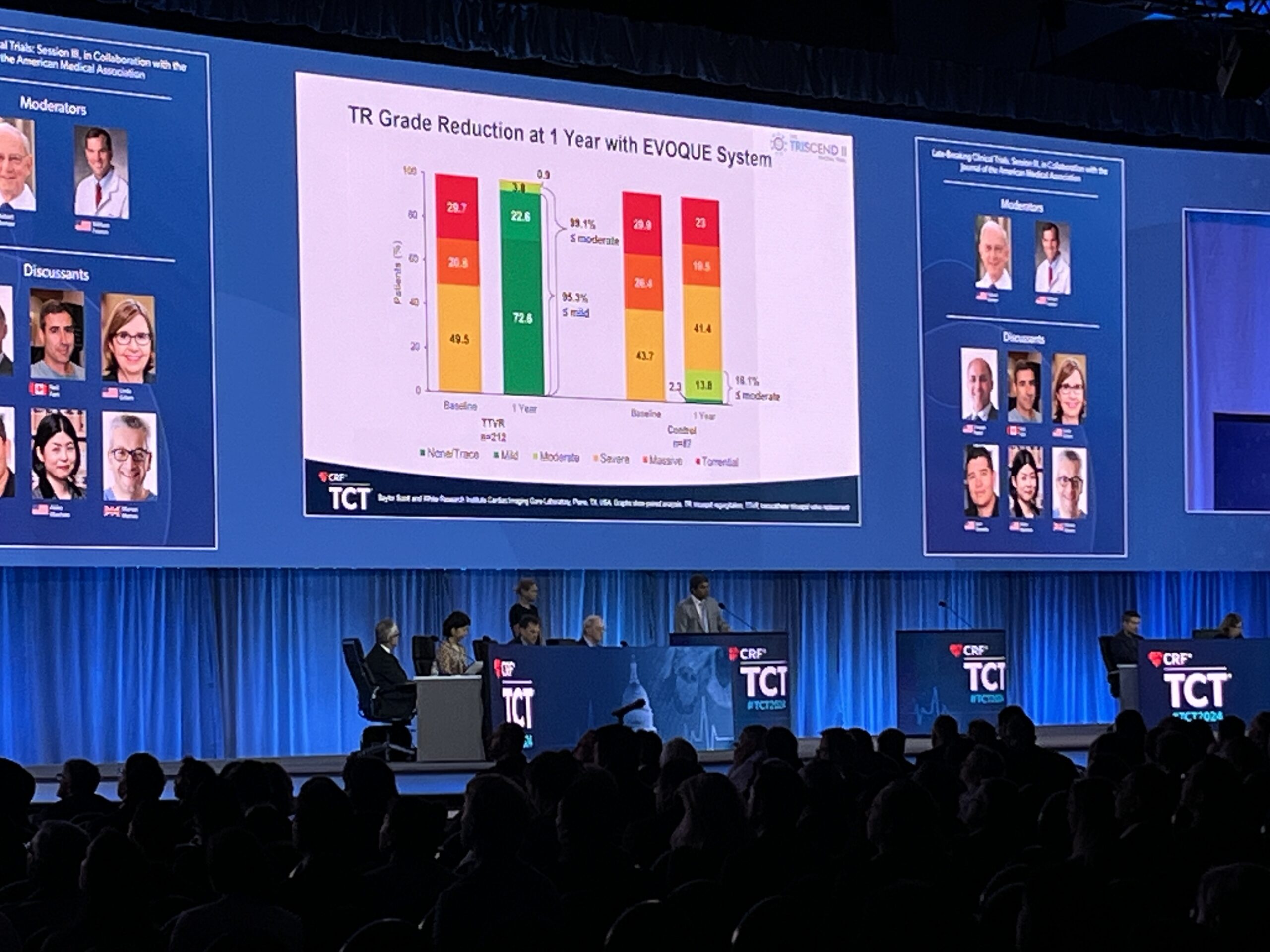
Patients enrolled in the TRISCEND II trial had at least severe tricuspid regurgitation (TR). The EVOQUE valve was successfully implanted in 95.4% of patients, and of those who received the valve, nearly all (95.3%) achieved almost complete TR elimination with ≤mild TR at one year, compared to 2.3% of patients receiving OMT alone. TR reductions were associated with significant improvements in symptoms, function and QoL at one year, with favorable numerical outcomes in mortality and heart failure hospitalisation.
“It is exciting to have the Evoque system available as a treatment option for patients who are very sick and otherwise have limited, if any, options,” said Kodali. “The one-year outcomes from the TRISCEND II trial demonstrate the benefits of this therapy in these patients and the favourable trends in all-cause mortality and heart failure hospitalisation are encouraging to see. We are pleased to see transcatheter tricuspid valve replacement reach this stage after nearly a decade of development.”
“The TRISCEND II trial results also demonstrated sustained quality-of-life benefits for patients receiving the EVOQUE system,” said Arnold. “Patients receiving TTVR with the EVOQUE system were twice as likely to be alive with a good quality-of-life at one year, compared with the control group.”
The EVOQUE system is approved for use in both Europe and the USA, including four valve sizes (44mm, 48mm, 52mm and 56mm), with the 56mm valve recently approved in the US.