One-year outcomes of the TRAVEL II study, evaluating the Lux-Valve Plus (Jenscare Scientific) transcatheter tricuspid valve replacement, were released at TCT 2024 (27–30 October, Washington, DC, USA), showing that LuX-Valve Plus system is safe and effective in achieving short delivery times, low composite event rates, significant tricuspid regurgitation (TR) reduction and improvement in functional and QoL (quality of life) metrics.
TRAVEL II aims to evaluate the long-term safety and efficacy of LuX-Valve Plus in the application on patients with severe TR and high surgical risk. The clinical trial enrolled 96 patients from 15 centres in China.
The average age of the patients was 71.35 years old with an average Society of Thoracic Surgeons (STS) score of 9.09%. Among those enrolled in the trial, 37.5% of the patients had prior left heart valve surgery/intervention, and 15.63% of them had pacemaker/implantable cardioverter-defibrillator (ICD) implanted before.
Patients were combined with multiple other comorbidities, which formed a poor baseline and increased the risks of surgical treatment or difficulties of other interventional therapies. The results showed that the device success rate was about 97%, and the average device operation time was around 35.56 minutes.
The safety indicators showed that the incidence of composite events remained low at 12.50%. Incidence of myocardial infarction (MI), acute liver failure, cardiovascular injury requiring surgical intervention, life-threatening haemorrhage were all 0%. All-cause mortality was 4.17%. Incidence of acute renal failure, severe paravalvular leakage, and conversion to surgical tricuspid valve replacement or tricuspid valvuloplasty were 1.04%, 2.08%, and 3.13% respectively. Incidence of new onset degree III AVB requiring permanent pacemaker implantation was only 2.08%.
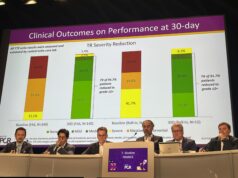
The efficacy indicators showed that the TR grade, New York Heart Assocation (NYHA) classification, and quality of life (QoL) improved significantly. At one year, 95.30% of patients showed no moderate or above TR.
In terms of NYHA cardiac function improvement, 85% of patients improved from pre-procedure NYHA class III/IV to class I/II. In terms of QoL, patients increased their Kansas City Cardiomyopathy Questionnaire (KCCQ) averaging score by 21 points. The results indicate that the cardiac function and QoL of patients improved continuously.
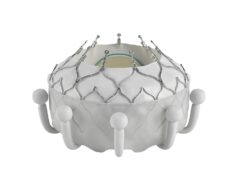
Juan F Granada, president and CEO of the Cardiovascular Research Foundation (CRF) concluded that LuX-Valve Plus system is a versatile transcatheter tricuspid valve replacement device that does not depend on radial force for anchoring.
Its design (ventricular septal anchor & leaflet-grasping clips) provides optional mechanisms for anchoring and stability. The multicentre, TRAVEL II study showed that LuX-Valve Plus system is safe and effective in achieving short delivery times, low one-year composite event rates, significant TR reduction and improvement in functional and QoL metrics at one year.