EARLY TAVR lived up to its billing as one of the most hotly anticipated trials to be presented at TCT 2024 (27–30 October, Washington, DC, USA), with spontaneous applause meeting the presentation of the trial’s primary endpoint result during Monday’s first late-breaking clinical trial session.
The positive outcome of the trial has led some to speculate that, instead of waiting for aortic stenosis symptoms to progress, physicians may now have the evidence to justify an early intervention in patients with evidence of severe aortic stenosis.
Conducted at 75 centres in the USA and Canada, EARLY TAVR looked at the safety and effectiveness of early intervention with transcatheter aortic valve implantation (TAVI) compared to clinical surveillance in patients with asymptomatic severe aortic stenosis.
Between March 2017 and December 2021 investigators, led by principal investigator Philippe Généreux (Morristown Medical Center, Morristown, USA), screened 1,578 patients for enrolment, ultimately randomising 901 patients to either TAVI (n=455) or surveillance (n=446). Patients enrolled had an average age of 76 and average Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score of 92.7, and were confirmed as asymptomatic through a protocol-mandated stress test and medical history evaluation.
The trial’s primary endpoint, a composite of death, stroke, or unplanned cardiovascular hospitalisation, was evaluated for superiority in the intent-to-treat population after a minimum follow-up of two years.
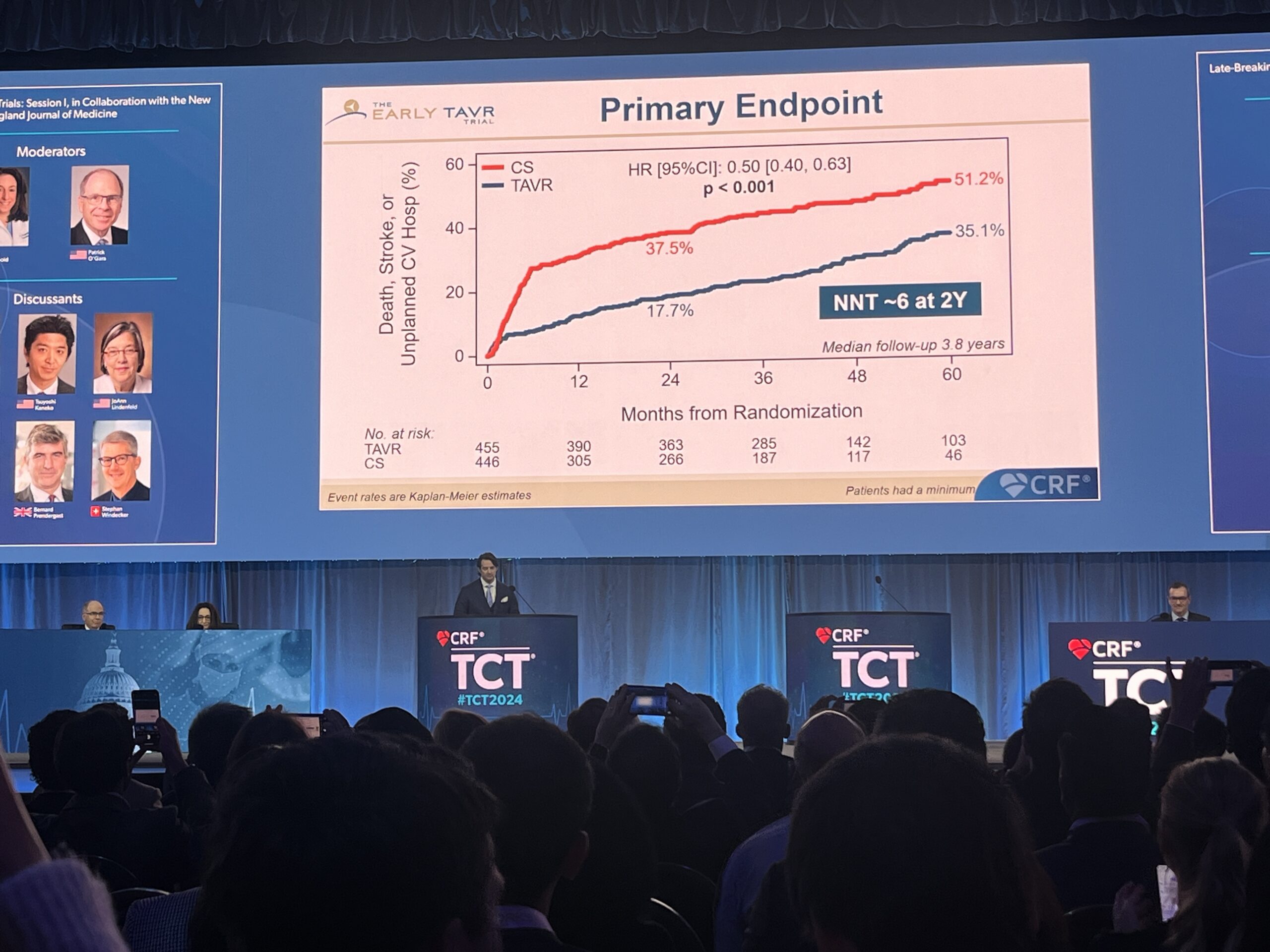
At TCT 2024, Genereux reported that early intervention with TAVI resulted in a significant reduction of the primary endpoint at two years as well as a median follow-up of 3.8 years, occurring 35.1% in the TAVR group compared with 51.2% in the surveillance group (p<0.001).
Breaking the result down further, he showed that the difference between the two strategies at a median follow-up of 3.8 years was predominantly driven by a difference in rates of unplanned hospitalisation seen in the two arms (20.9% for TAVI vs. 41.7% for surveillance) whilst rates of all-cause death (8.4% vs. 9.2%) and stroke (4.2% vs. 6.7%) were relatively similar in the two arms, albeit favouring TAVI in both metrics.
Additionally, Généreux reported that within the first six months, 26.2% of patients in the clinical surveillance arm converted to aortic valve replacement (AVR) with many presenting progressive or advanced symptoms. In the 12-month follow-up period after randomisation, the rate of conversion to AVR was 47.2%.
“Given the benefits observed and the lack of harm, early TAVI may be preferred to clinical surveillance in patients with asymptomatic severe aortic stenosis, especially when combined with the challenges of timely symptom recognition and prompt treatment in real-world settings,” Généreux said of the clinical implications of the trial.













