The SENTINEL trial, which was presented at the 2016 Transcatheter Cardiovascular Therapeutics (TCT) meeting (29 October-2 November, Washington , DC, USA) today, has not meet its primary efficacy endpoint that patients undergoing transcatheter aortic valve implantation (TAVI) with a cerebral protection device (Sentinel, Claret Medical) would have a significant reduction in new lesion volume compared with those undergoing TAVI without the device. However, the primary efficacy endpoint has been met in a multivariate model.
The prospective multicentre randomised trial included 363 patients with severe symptomatic aortic stenosis and planned TAVI who were at high risk for surgery at 17 centres in the US and two centres in Germany. Patients were randomised 1:1:1 into a safety arm (Sentinel device arm only) and two imaging cohorts, in which patients were randomly assigned to TAVI with the Sentinel device or TAVI without the device. Blinded diffusion weighted MRI (DW-MRI) and neurocognitive function assessments were performed in the device and control arms. Particulate debris from the extracted filters was studied in the device arm and all patients underwent rigorous neurologic evaluations post-TAVI, at 30 days and at 90 days.
The median total new lesion volume in protected territories (the primary endpoint) was not significantly different in the device arm compared with the control arm (102.8 mm3 vs. 178.0 mm3, P=0.33). However, there were important confounders identified including transcatheter valve type and baseline MRI lesion volume (marker of baseline cerebral disease burden). After adjusting for these factors, embolic protection resulted in reduction of new lesion volume on MRI.
The primary safety endpoint was the occurrence of major adverse cardiac and cerebrovascular events (MACCE) at 30 days compared to a historical performance goal. The study found MACCE (7.3%) in the device and safety arms was non-inferior to the performance goal (18.3%, P noninferior <0.001) but was not statistically different from the control (9.9%, P=0.41). Despite the finding of histopathologic debris within filters in 99% of patients, which included thrombus, calcification, valve tissue, artery wall and foreign material, all strokes at 30 days were not significantly different in the device and safety arms versus the control arm (5.6% vs. 9.1%, P=0.25). However, study presenter Susheel Kodali (NewYork-Presbyterian Hospital/Columbia University Medical Center, New York, USA) noted that the study was not powered for clinical endpoints. Furthermore while there was no significant improvement in neurocognitive function associated with embolic protection, there was a correlation discovered between new lesion volume and neurocognitive decline (P=0.0022).
Kodali commented: “Despite not meeting its primary efficacy endpoint, the study does provide evidence of device safety and confirms the high-frequency of embolic debris capture with the Sentinel dual filter neuroprotection therapy. In addition, there are important lessons from this trial which should impact future research on neuroprotection during TAVI.”
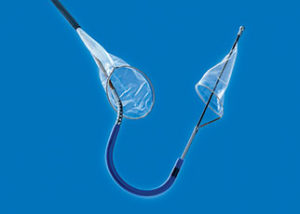
The Sentinel transcatheter cerebral embolic protection device consists of two filters within a single six French delivery catheter percutaneously placed from the right radial (preferred) or brachial artery over a 0.014” guide wire. The filters are positioned in the brachiocephalic and the left common carotid arteries before TAVI and are withdrawn into the catheter and removed after TAVI.
As well as being presented at TCT, the study was simultaneously published in the Journal of the American College of Cardiology.










