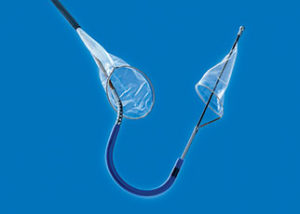
Data from the SENTINEL pivotal IDE trial will be presented during a late-breaking trial session at the 2016 Transcatheter Cardiovascular Therapeutics (TCT) meeting (29 October–2 November, Washington, USA). The trial evaluated Claret Medical’s Sentinel cerebral protection system (CPS)—according to a press release, the only device designed to protect the brain by capturing and removing embolic debris dislodged during transcatheter aortic valve implantation (TAVI).
The SENTINEL results will be presented by co-principal investigator Susheel Kodali Columbia University Medical Center/New York-Presbyterian Hospital, New York, USA) in the main arena at 9am on 1 November. The trial is a prospective, randomised, controlled, blinded study of 363 TAVI patients at 19 centres in the US and Germany, and its endpoints included reduction in new ischaemic cerebral infarcts, major adverse cardiac or cerebrovascular events, neurocognitive outcomes, and quantitative and qualitative histopathological findings. It allowed inclusion of all TAVI platforms commercially available in the USA.
Claret Medical president and chief executive officer Azin Parhizgar says: “The importance of this landmark trial in highlighting the impact of TAVI on the unprotected brain of patients is acknowledged by its acceptance as part of the TCT late-breaking clinical trial session. We look forward to sharing new scientific insights from this rigorous trial, as we develop a large collection of clinical evidence on the impact of ischemic cerebral infarcts and the protective benefits that the Sentinel CPS may provide.”
The device will also be featured in several other presentations at the TCT conference:
- Is Cerebral Embolic Protection Needed for TAVR? The Evidence from DEFLECT 3, CLEAN-TAVI, and the SENTINEL Clinical Trials, presented by Samir Kapadia: 31 October 2pm (Room 152, Level 1)
- Claret Medical Symposium—SENTINEL Science: Compelling New Evidence for Cerebral Protection in TAVR. 1 November 1–2pm (presentation Theater 5). Symposium co-chairpersons include Martin Leon, Axel Linke and Adnan Siddiqui.
- FDA Town Hall Session—Spectrum and Assessment of TAVR Accessory Devices, presented by Martin Leon: 1 November 3:25pm (room 202, Level 2)
- Cerebral Embolic Protection I: The Sentinel Filter—design features and updates from CLEAN-TAVI, SENTINEL RCT and others, presented by Axel Linke: 1 November; 5:45pm (room 152, Level 1)
The Sentinel CPS received the CE Mark in 2013. More than 3,000 patients have been protected by a Claret Medical cerebral protection system.










