Transcatheter aortic valve implantation (TAVI) was no better than a conservative, surveillance strategy in preventing all-cause death, disabling stroke, hospitalisation and Kansas City Cardiomyopathy Questionnaire (KCCQ) score in patients with systolic heart failure and moderate aortic stenosis.
This was the headline finding of the TAVR UNLOAD study, in which investigators sought to determine whether TAVI may provide clinical benefit in patients with heart failure with reduced ejection fraction (HFrEF) and concomitant aortic stenosis, complementary to guideline-directed medical therapy.
Finidings of the trial, which randomised 178 patients at 66 centres in USA, The Netherlands and Austria 1:1 to either TAVI or surveillance between January 2017 and December 2022, were presented during a late-breaking trial session at TCT 2024 by Nicolas van Mieghem (Thoraxcenter, Erasmus University Medical Center, Rotterdam, The Netherlands) and published simultaneously published in the Journal of the American College of Cardiology (JACC).
“Medical neurohormonal modulation and afterload reduction are key elements in the treatment of HFrEF,” Van Mieghem and colleagues write in their JACC paper, setting out the rationale for the trial. “Degenerative aortic stenosis increases with ageing and contributes to the overall valvuloarterial impedance in patients with HFrEF; treatment of aortic stenosis may therefore represent a complementary target for afterload reduction in this population.”
Current guidelines recommend TAVI for symptomatic severe aortic stenosis or for asymptomatic severe aortic stenosis with left ventricular (LV) ejection fraction <50%, but not for moderate aortic stenosis.
TAVR UNLOAD was an investigator-initiated, international, randomised controlled, open label, superiority trial comparing transfemoral TAVI with clinical aortic stenosis surveillance and aortic valve replacement upon progression to severe aortic stenosis in symptomatic patients with HFrEF and moderate stenosis.
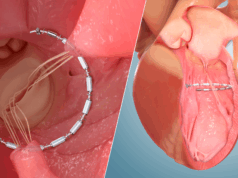
Investigators enrolled patients with New York Heart Association (NYHA) class II-IV HFrEF (LV ejection fraction [LVEF] ≥20% and <50) and moderate aortic stenosis, determined as an aortic valve area >1cm2 and ≤1.5 cm2 who were eligible for transfemoral TAVI with a balloon-expandable, Sapien 3 (Edwards Lifesciences) valve.
The original study design required 600 patients (two groups of 300) with a primary endpoint analysis at one year. However, an updated protocol allowed for the use of the longest available follow-up data, justifying a reduced sample size of 300 patients (2 groups of 150). Due to funding limitations and slow enrolment, the Steering Committee decided to terminate enrolment by 31 December 2022, ensuring at least one year of follow-up for all participants.
Van Mieghem detailed that the enrolled patient population had a mean age of 77 years, 20.8% were female, 55.6% were in NYHA class III or IV, and the mean LVEF was 39%. A total of 35 patients in the medical therapy group, 39% of the cohort, progressed from moderate to severe aortic stenosis during the trial.
The primary endpoint was the hierarchical occurrence of all-cause death, disabling stroke, disease-related hospitalisations, and heart failure equivalents, as well as the change from baseline in the Kansas City Cardiomyopathy Questionnaire Overall Summary score (KCCQ-OS).
Investigators used the Finkelstein-Schoenfeld to analyse the treatment effects of the two approaches, which demonstrated that, at one year, TAVI resulted in more wins driven by clinically meaningful improvement in quality of life compared with aortic stenosis surveillance.
However, TAVI did not affect the primary composite endpoint at a median follow up of 23 months. In addition, major adverse cardiovascular and cerebrovascular events (MACCE) and all-cause death were not significant between the two groups.
During his presentation, Van Mieghem detailed a number of limitations of the study, commenting that it was underpowered due to slow enrolment and insufficient sample size and that study design changes affected the study results.
“Although TAVI for moderate aortic stenosis in patients with systolic heart failure on guideline-directed medical therapy was safe, it did not affect the primary hierarchical composite endpoint at a median follow up of 23 months,” said Van Mieghem.