Tag: 510(k)
FFRangio system receives US FDA clearance
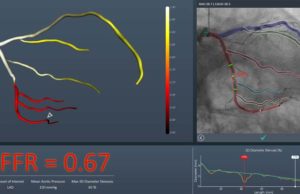
US FDA 510(k) clearance has been granted for the FFRangio System (CathWorks). The FFRangio syste...
First patients treated in United States with OrbusNeich Teleport Microcatheter
The first patients in the United States have been treated using the OrbusNeich Teleport Microcathete...