Pi-Cardia has announced the successful completion of its ShortCut pivotal study enrolment.
Pi-Cardia has announced the successful completion of its ShortCut pivotal study enrolment.
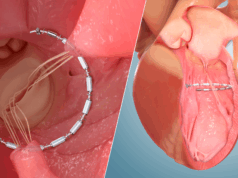
ShortCut is designed to split the leaflets of pre-existing valves to enable safe transcatheter aortic valve replacement (TAVI) in patients at risk of coronary obstruction.
The study was conducted across 20 of the world’s leading TAVI centres in the USA and Europe and was led by Susheel Kodali (New York Presbyterian/Columbia University, New York, USA), Didier Tchétché (Clinique Pasteur, Toulouse, France) and Danny Dvir (Sha’are Zedek, Jerusalem, Israel). It included 60 patients undergoing valve-in-valve procedures, in whom ShortCut was used as a preceding step to TAVI implantation. Data was independently analysed by central echo and computed tomography (CT) core-labs and baseline data will be presented at the upcoming TCT meeting.
The ShortCut device is designed to address a fundamental challenge in TAVI, that the leaflets of pre-existing valves are pushed sideways, compromising normal flow in the sinuses and hindering coronary access. The device enables the pre-existing valve leaflets to be split, facilitating a safer valve-in-valve procedure and potentially broadening the TAVI market’s reach to younger patients.
“We are excited to have been able to complete enrolment in such a groundbreaking study so efficiently,” said Kodali. “It clearly demonstrates the unmet need for ShortCut and how excited the sites were to learn and perform the procedure. I look forward to the commercial launch of ShortCut in the USA in the near future.”
“We have spent years looking for techniques to overcome the life-threatening complication of coronary obstruction,” said Dvir. “Having performed multiple ShortCut cases, I can clearly say we have found a simple, controlled and teachable solution that integrates well into the workflow of every TAVI centre.”
“I can see ShortCut becoming instrumental, not only in facilitating safe valve-in-valve procedures, but also for ensuring future coronary access in patients receiving their first TAVI or optimising TAVI in bicuspid valves,” said Tchétché. “Lifetime management for patients with aortic stenosis is increasingly important as we treat younger patients. ShortCut holds the key for TAVI to safely expand to these large populations.”
ShortCut is part of Pi-Cardia’s leaflet modification product portfolio, which includes the ShortCut Mitral for splitting leaflets in patients at risk for left ventricular outflow tract obstruction following transcatheter mitral valve replacement (TMVR), and Leaflex, a standalone, non-implant-based mechanical scoring device to restore leaflet mobility and improve haemodynamics for patients with aortic stenosis. Leaflex global clinical trials are underway.
“We are thrilled having completed enrolment months ahead of schedule,” said Erez Golan, Pi-Cardia’s chief executive officer. “This milestone not only validates our market models predicting a significant percentage of TAVI patients in need for leaflet modification, but it also means we should be able to commercialise ShortCut in the USA and Europe as soon as 2024.”