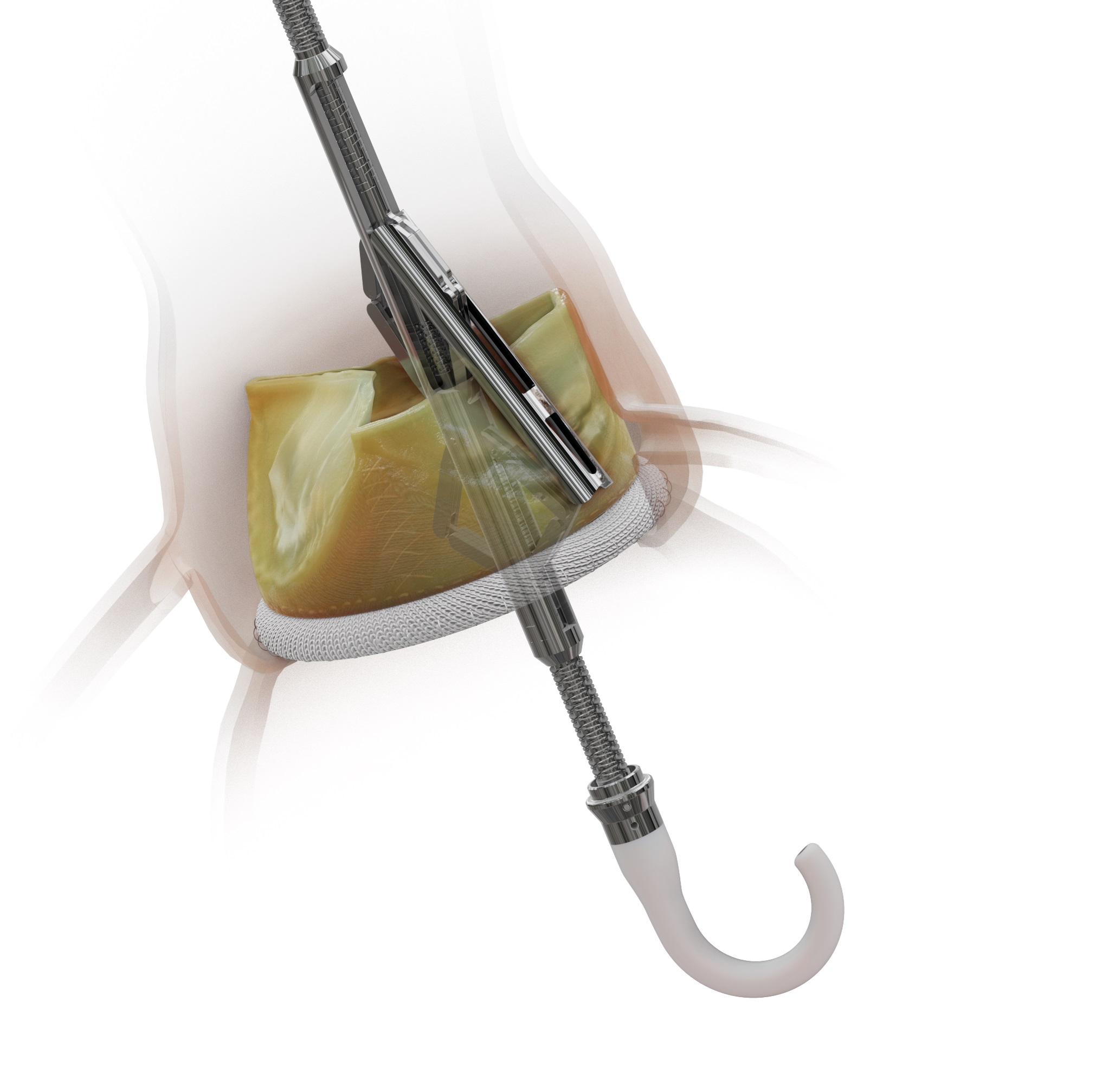
The US Food and Drug Administration (FDA) has provided market clearance for the ShortCut (Pi-Cardia) dedicated leaflet modification device, which is designed to enable valve-in-valve transcatheter aortic valve implantation (TAVI) procedures in patients at risk of coronary obstruction.
“Lifetime management of aortic stenosis calls for leaflet modification solutions like ShortCut to ensure that we are carefully addressing the risk of coronary obstruction before implanting a valve,” said Martin B Leon (New York-Presbyterian/Columbia University Medical Center and Columbia University College of Physicians and Surgeons, New York, USA) who chairs the global steering committee for ShortCut studies.
“The rigorous pivotal study leading to this important market clearance by the FDA demonstrates that ShortCut was both safe and effective in achieving the intended leaflet split in all patients. Importantly—it also shows that mechanical splitting with ShortCut, in both single- and dual-leaflet cases, was a controlled and teachable procedure, making it adoptable by TAVI centres as a critical step pre-implantation, so that patients at risk of coronary obstruction may be safely treated, without disruption of TAVI workflow.”
As bioprosthetic valves degenerate over time, patients will at some point likely need a valve-in-valve procedure to be performed, and a significant portion of them who are at risk for coronary obstruction will require leaflet splitting with ShortCut, Pi-Cardia says in a press release.
Recent models published by Philippe Généreux (Morristown Medical Center, Morristown, predict that by 2035, more than 42,000 valve-in-valve procedures will be performed annually in the USA, representing ∼15% of all TAVI procedures. Future planned indication expansion into native and bicuspid valves may mean that around 30% of future TAVI cases will require leaflet modification with ShortCut in order to be performed safely and obtain optimal results for patients, Pi-Cardia’s press release adds.
“We are excited and immensely proud to introduce the first dedicated leaflet modification device to the US market,” said Erez Golan, chief executive officer of Pi-Cardia. “With FDA clearance now in hand, we are focused on building a strong commercial and clinical support team to execute a limited commercial launch while ensuring optimal patient outcomes. Building on the breakthrough device designation awarded to ShortCut earlier this year, we are committed to collaborating closely with our physician and hospital system partners to ensure they have access to this unique enabling device. ShortCut clearance by the FDA opens a new era in the lifetime management of patients with aortic stenosis.”