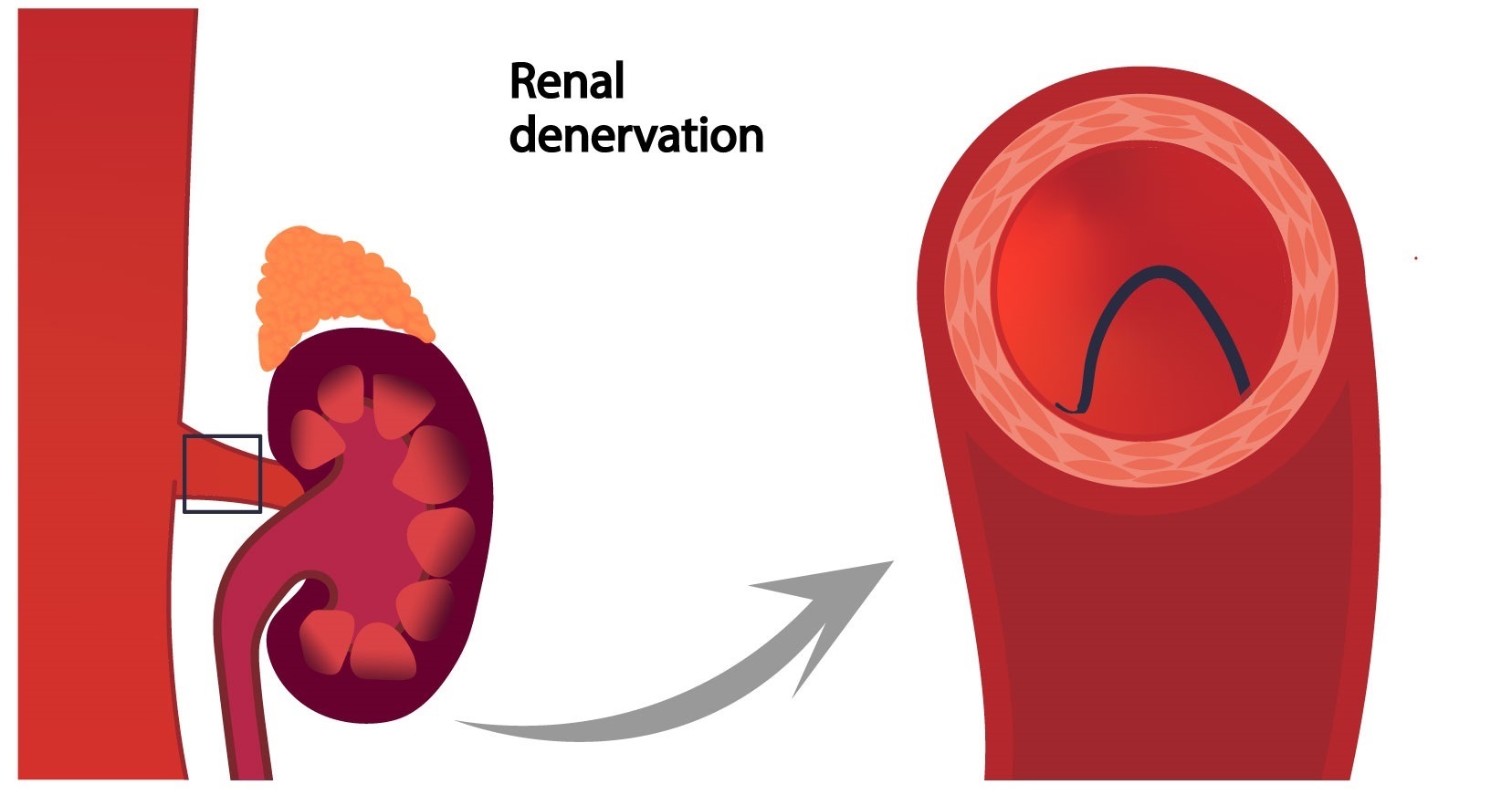
 The approval of two renal denervation devices by the US Food and Drug Administration (FDA)—the Paradise (Recor Medical) and Symplicity Spyral (Medtronic) systems—represent a “groundbreaking advancement in the treatment of patients with uncontrolled hypertension” the Society for Cardiovascular Angiography & Interventions (SCAI) has stated.
The approval of two renal denervation devices by the US Food and Drug Administration (FDA)—the Paradise (Recor Medical) and Symplicity Spyral (Medtronic) systems—represent a “groundbreaking advancement in the treatment of patients with uncontrolled hypertension” the Society for Cardiovascular Angiography & Interventions (SCAI) has stated.
The remarks come in a statement issued shortly after the announcement of the receipt of approval for the radiofrequency-based Symplicity Spyral system late last week, just days after it was announced that the ultrasound-based Paradise system had become the first such device to receive approval in the USA.
“The approval of the renal denervation systems by the FDA is a game-changer for both interventional cardiology and the treatment of hypertension,” George Dangas (Icahn School of Medicine at Mount Sinai, New York, USA), president of SCAI, was quoted as saying. “This innovative technology has the potential to revolutionise how we approach the management of high blood pressure which has grown tremendously globally, offering patients a safe and effective treatment option.”
In the summer, SCAI released a position statement on renal denervation for hypertension regarding patient selection, best practices for optimal techniques, competence, training and organizational recommendations. The European Society of Hypertension (ESH) also updated its own arterial hypertension management guidelines in which it stated that renal denervation can be proposed as an adjunctive therapy in select patients with resistant hypertension.