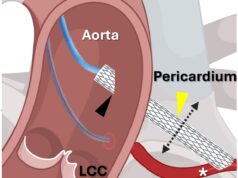
OpSens has announced the inclusion of SavvyWire in the COMPLETE TAVR clinical study to investigate the impact of standardised invasive haemodynamics (SIH) during transcatheter aortic valve implantation (TAVI) procedures.
OpSens has announced the inclusion of SavvyWire in the COMPLETE TAVR clinical study to investigate the impact of standardised invasive haemodynamics (SIH) during transcatheter aortic valve implantation (TAVI) procedures.
COMPLETE TAVR, an investigator-initiated study sponsored by Edwards Lifesciences, will determine whether a strategy of complete revascularisation involving staged percutaneous coronary intervention (PCI) using drug-eluting stents to treat all suitable coronary artery lesions after successful balloon expandable TAVI, is superior to a strategy of medical therapy alone in reducing the composite outcome of cardiovascular death, new myocardial infarction, ischaemia-driven revascularisation or hospitalisation for unstable angina or heart failure.
The COMPLETE TAVR study is a randomised, multicentre, open-label trial with blinded adjudication of outcomes with planned enrolment of 4,000 patients at up to 120 centres. The SIH sub-study using the SavvyWire will enroll up to 200 patients at up to 20 centres across the USA and Canada.
“We look forward to the results of the COMPLETE TAVR standardized invasive haemodynamics study, where SavvyWire has the potential to simplify both the acute and long-term haemodynamic assessment of patients post-TAVR,” said David Wood, Director, UBC Centre for Cardiovascular Innovation (Vancouver, Canada) and global principal investigator of the COMPLETE TAVR study.
The SIH sub-study using SavvyWire has already started enrolment, and is anticipated to be completed later in 2023, with results anticipated early in 2024.