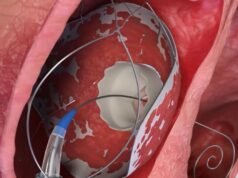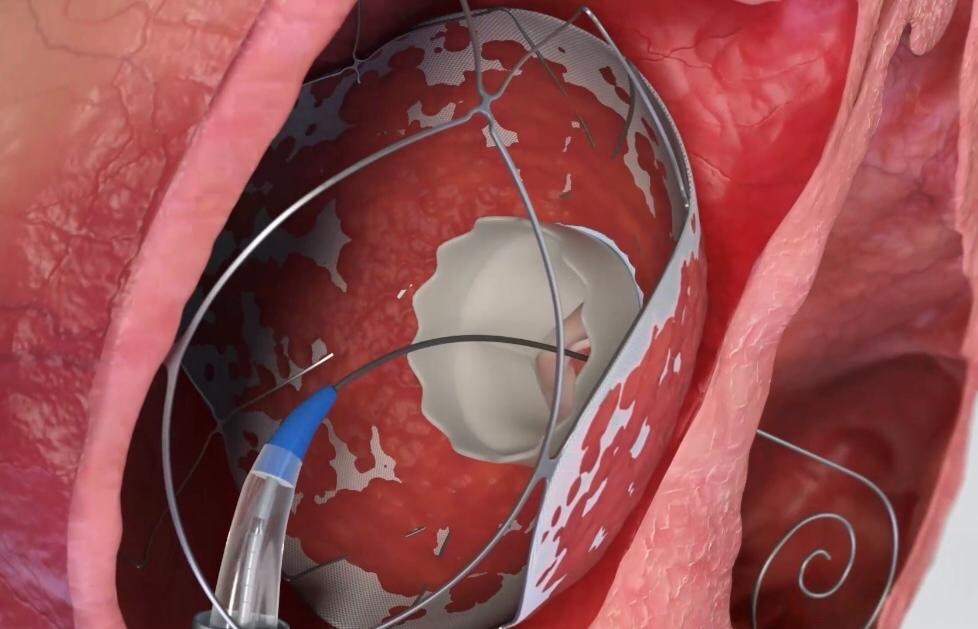
Allmed Solutions has announced first-in-human use of the RoseDoc system, a two-stage heart valve replacement system developed by its subsidiary TruLeaf Medical. The new technology allows for full replacement of a diseased heart valve to be fully replaced through a needle stick access point.
The procedure was carried out on two patients suffering from severe heart failure caused by a massive leak in their tricuspid valve. For both patients, conventional medical and surgical options had been exhausted, and their condition was considered too severe to undergo standard open-heart surgery. The investigational treatment was therefore provided under a compassionate care pathway.
The replacement was completed in two stages. Approximately three months prior, a novel proprietary docking system was implanted in the patients’ hearts. The final procedure, completed recently, involved the implantation of the valve itself—the RoseDoc system.
According to a press release issued by Allmed Solutions, restoration of normal valve function resulted in immediate and dramatic clinical improvement. Both patients reported a substantial change in their functional capacity and were discharged home within five days of the final valve implantation.
Oz Shapira, chief executive officer of Allmed Solutions, said: “The success recorded in recent days is a defining moment. The ability to fully replace heart valves via needle stick only, with no incisions and no opening of the chest, is an extraordinary breakthrough. We are entering a new and safer era for patients.”
Shapira said that the main innovation offered with RoseDoc is its docking system, which enables the catheter-based replacement of two different types of heart valves, both the tricuspid valve on the right side and valves on the left side of the heart.
TruLeaf Medical was founded in 2017 by three Israeli entrepreneurs: Benjamin Spenser, Nathanael Benichu, and the late Uri Rosenstein. The team had previously developed the first transcatheter valve replacement, the Sapien 3 valve, which was part of PVT, a company later acquired by the global medical-device giant Edwards Lifesciences.
Following the successful early trial, the company is continuing to recruit patients in India and plans to expand the trial to sites in Israel, South Africa, and Uzbekistan as it advances toward global regulatory approval and commercialisation.