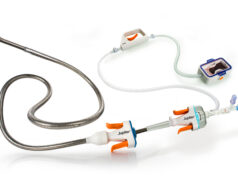
PulseCath has announced the transition from CE marking to EU Medical Device Regulation (MDR) certification for its iVAC 2L percutaneous mechanical circulatory support (MCS) device.
PulseCath has announced the transition from CE marking to EU Medical Device Regulation (MDR) certification for its iVAC 2L percutaneous mechanical circulatory support (MCS) device.
iVAC 2L can be used to facilitate high-risk percutaneous coronary interventions (PCI), pumping blood from the left ventricle to the aorta, synchronising with the natural rhythm of the patient’s cardiac cycle. It enables the intervention to be performed with a circulatory back-up that keeps the patient hemodynamically stable.
The device is inserted through the femoral artery. The tip of the catheter containing the inlet is positioned in the left ventricle and the outlet valve is placed at the height of the ascending aorta, just at the level of the coronary ostia. The external pump is then activated, which synchronises with the patient’s electrocardiogram or aortic pressure signal, and pumps the blood for the duration of the entire treatment, aspirating the blood during systole and ejecting it to the ascending aorta during diastole. Once the high-risk treatment is over, the ventricular support is removed.
Oren Malchin, CEO of PulseCath, said: “This achievement marks a significant milestone for PulseCath and underscores our commitment to delivering innovative solutions that meet the highest regulatory standards. iVAC 2L represents a leap forward in complex high-risk PCI’s, and we are eager to make this transformative device available to healthcare providers and patients worldwide.”