Details of the first pivotal trial assessing the use of a dedicated leaflet splitting device to facilitate future coronary access in patients undergoing transcatheter aortic valve implantation (TAVI) who may be at risk of valve-in-valve induced coronary obstruction were shared at PCR London Valves (19–21 November, London, UK).
ShortCut (Pi-Cardia) is designed to split the leaflets of pre-existing valves to enable coronary access and prevent obstruction during TAVI procedures in failed bioprosthetic surgical (valve-in-valve) or TAVI valves (TAV-in-TAV). The device is delivered transfemorally over a routine left ventricular wire and once it reaches the target area at the base of the leaflet a small blade is advanced which, once manually pulled through, creates a split in the leaflet.
“There are a growing number of patients available for TAVI due to the expansion of indications and the eligibility of younger patients,” said Didier Tchétché (Clinique Pasteur, Toulouse, France) of the current outlook for the technology in a presentation detailed the latest status of the ShortCut pivotal trial during a late-breaking trials session at PCR London Valves.
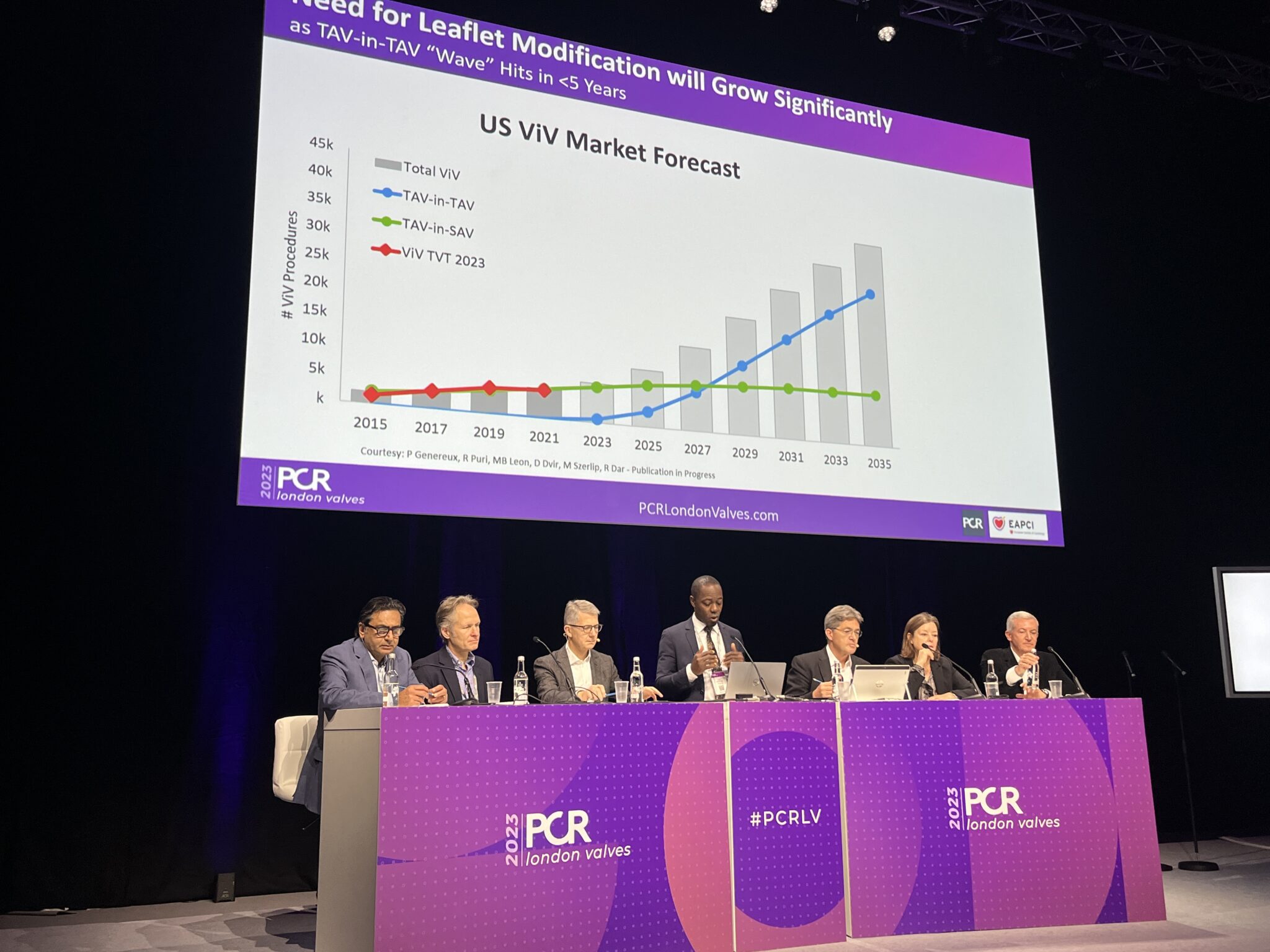
“These patients will require redo interventions in the future, and we know from US market forecasts that TAV-in-TAV is going to be more frequent than valve-in-valve in the future. In 2035 see that there is going to be a six-fold increase in the number of valve-in-valve procedures, [so] there will be need for leaflet modification techniques.”
The pivotal study of the device which is the first to be designed specifically to address the need for leaflet splitting, has enrolled 60 patients at sites in USA, Europe and Israel. The trial was designed to test safety, including the occurrence of mortality and stroke up to 30 days, and efficacy of the procedure assessed intraprocedurally by transoesophageal echocardiography as well as freedom from coronary obstruction or reintervention out to 30 days.
Patients have been included in the study if they were undergoing a valve-in-valve procedure at deemed to be at risk of coronary obstruction based upon a heart team decision. Tchétché described the trial population as being at “high-risk”, with a mean age of 77 and an average Society of Thoracic Surgeons (STS) risk score of 4, with a high number of comorbidities.
There was a large variety of different devices that were seen during the trial, Tchétché detailed. “I would say that you could split any kind of failed bioprosthesis, whether it be stented, non-stented, externally mounted leaflets, or even TAVI devices,” he commented. The majority of cases involved failed surgical bioprosthesis, but some TAV-in-TAV procedures were also included.
Risk of coronary obstruction was determined by virtual valve-to-coronary (VTC) and valve to sinotubular junction (VTS) distances, which stood at an average of 3.3mm and 2.2mm respectively. Dual leaflet splitting was required in 37% of cases. “We are going to see more and more in the future effective controlled splitting of single or double leaflets,” Tchétché commented, adding that the procedural times seen in the trial were on average 30–47 minutes.
Results of the trial are expected upon completion of follow-up, which is likely to be in 2024, Tchétché noted.