 Teleflex has announced that the preliminary results from its Ringer perfusion balloon catheter (PBC) investigational device exemption (IDE) study were reported in a featured presentation at the CTO Plus conference (28 February–1 March, New York, USA) by the study’s principal investigator, David Kandzari (Piedmont Heart Institute, Atlanta, USA).
Teleflex has announced that the preliminary results from its Ringer perfusion balloon catheter (PBC) investigational device exemption (IDE) study were reported in a featured presentation at the CTO Plus conference (28 February–1 March, New York, USA) by the study’s principal investigator, David Kandzari (Piedmont Heart Institute, Atlanta, USA).
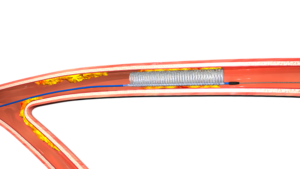
Ringer PBC is a rapid-exchange percutaneous transluminal coronary angioplasty (PTCA) catheter with a unique helical balloon. When inflated, the balloon approximates a hollow cylinder with a large central perfusion lumen, allowing for continuous coronary blood flow during prolonged inflations.
The Ringer PBC study is a limited prospective, multicentre, single-arm IDE study, undertaken at four sites in the USA investigating the Ringer PBC for the management of emergent coronary perforations that develop during percutaneous coronary intervention (PCI) procedures.
The study enrolled 30 participants, and analysis was performed based upon intention-to-treat. The primary efficacy endpoint required successful Ringer PBC delivery and inflation at the perforation site, control of extravasation (defined as residual Ellis grade 0 or 1), and preservation of antegrade coronary flow (Thrombolysis in Myocardial Infarction [TIMI] grade 2 or 3).
Of 30 participants enrolled, the primary efficacy endpoint was observed in 22 participants (73.3%), with successful Ringer PBC delivery in 26 participants (86.7%). Among those participants with successful delivery, control of extravasation with perfusion was achieved in 22 participants (84.6%). Twelve participants were treated with a covered stent following perforation management with the Ringer PBC. One participant required emergency surgery for complications of pericardiocentesis, and three participants died despite control of extravasation from the index perforation.
“I believe these preliminary study results are important,” said Kandzari. “Treatment options for patients with coronary artery perforations during PCI cases have been limited to date, and this trial points the way to developing dedicated devices.”
For the investigational use of the management of coronary perforations, Ringer PBC was granted US Food and Drug Administration (FDA) breakthrough device designation, a program intended to provide patients and healthcare providers with timely access for certain medical devices that could potentially provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions by speeding up development, assessment, and review for premarket approval, 510(k) clearance, and de novo marketing authorisations.
“Teleflex is committed to generating the clinical evidence to help physicians make confident decisions when selecting the right products for their patients’ needs,” said Teleflex medical director, Christopher Buller.
Ringer PBC is currently indicated for balloon dilatation of coronary artery or coronary bypass graft stenoses where the physician desires distal blood perfusion during balloon inflation for the purpose of improving myocardial perfusion. The Ringer PBC in this study is an investigational device and not available for sale. Data from the study is intended to support a premarket application recently submitted to FDA.









