Vivasure Medical has enrolled the 100th patient in its PATCH clinical study, a multicentre, single-arm, pivotal study evaluating the safety and efficacy of the Vivasure PerQseal closure device system.
The US Food and Drug Administration (FDA) granted investigational device exemption (IDE) approval earlier this year to advance the PATCH study forward.
“We are grateful to the one hundred patients who have been enrolled in this study to date and the brilliant physician investigators working to gather data about our technology,” said Andrew Glass, chief executive officer of Vivasure Medical. “This marks an important milestone for Vivasure as it brings us closer to our goal of demonstrating PerQseal’s potential in supporting successful percutaneous cardiovascular therapies.”
Large hole arterial access is required for clinicians to perform numerous percutaneous cardiovascular procedures such as transcatheter aortic valve implantation (TAVI), thoracic and abdominal endovascular aneurysm repair (TEVAR and EVAR), and the use of a cardiac assist device (CAD).
The current approach to large-diameter arterial closure is a surgical repair or the use of suture- or collagen-based closure devices. Both can result in major vascular complications, such as vessel distortion at the closure site, which may lead to stenosis, thrombus formation or abrupt closure.
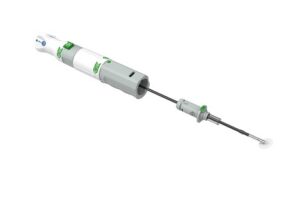
PerQseal is designed as a sutureless, fully absorbable synthetic implant for large-bore vessel punctures. Its low profile patch can be placed from inside the vessel and is intended to make deployment simpler and more controlled than conventional closure techniques. Clinical studies to date have shown a low complication rate and high technical success.
The PATCH pivotal study will enrol and follow up to 188 patients across the USA and Europe. The company intends to use the clinical study results to support an FDA premarket approval submission as well as multinational commercial launch of the PerQseal system for large hole vessel closure.










