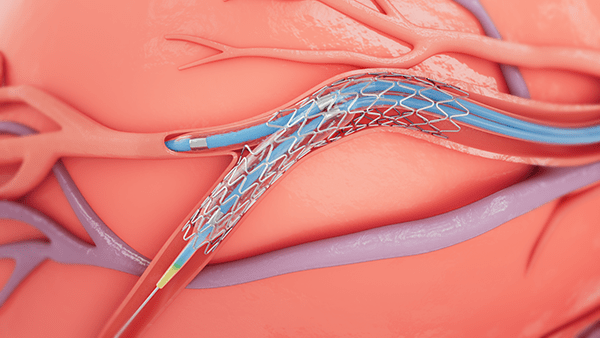
 Medtronic has received Food and Drug Administration (FDA) approval for the treatment of non-left main bifurcation lesions utilising the provisional bifurcation stenting technique—using a single stent to treat the bifurcation—with the Onyx Frontier and the Resolute Onyx drug-eluting stent (DES) systems.
Medtronic has received Food and Drug Administration (FDA) approval for the treatment of non-left main bifurcation lesions utilising the provisional bifurcation stenting technique—using a single stent to treat the bifurcation—with the Onyx Frontier and the Resolute Onyx drug-eluting stent (DES) systems.
This indication will allow Medtronic to provide a robust portfolio of medical education and procedural training for physicians performing percutaneous coronary interventions (PCIs) in patients with bifurcation lesions, the company said in a press release.
Bifurcation lesions occur when plaque builds up around the junction of two coronary arteries. These lesions are considered challenging to treat because of anatomical variations in the vessels and the difficulty associated with reaching the side branches. The treatment of bifurcation lesions is commonly associated with lower success rates and increased rates of long-term adverse cardiac events.
“The bifurcation expanded indication is yet another exciting milestone for our coronary business this year,” said Jason Weidman, senior vice president and president of the Coronary & Renal Denervation business, which is part of the Cardiovascular Portfolio at Medtronic. “As the first and only medical device company to offer this indication to US interventional cardiologists, Medtronic remains committed to investing in DES technology, clinical evidence, and physician education. We are looking forward to helping even more physicians access the tools they need to give their patients best-in-class care.”
Both the Onyx Frontier and Resolute Onyx DESs are FDA and CE mark approved.










