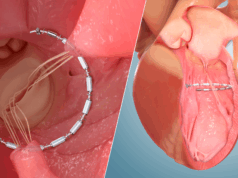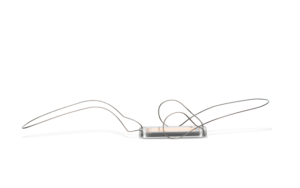
Endotronix has announced the one-year clinical results from PROACTIVE-HF, evaluating outcomes for the Cordella pulmonary artery (PA) sensor system.
The data were shared as part of the Heart Failure Society of America (HFSA) annual scientific meeting, which had been due to take place 27–30 September in Atlanta, USA, but had been cancelled due to Hurricane Helene. Late-breaking studies that had been due to be presented in-person at the meeting were live-streamed online.
The data demonstrated that the 456 patients managed with Cordella in the study experienced a meaningful reduction in one-year heart failure (HF) hospitalisation and all-cause mortality rate (36 events per 100 patients versus the 70 events per 100 patients that were pre specified; 49% lower).
The study’s secondary endpoints of quality of life and biomarker reduction also showed significant improvements: 10.5% in the Kansas City Cardiomyopathy Questionnaire (KCCQ 5.7 points); 13.3% in the 6-minute walk test (35 meters); 36.2% in New York Heart Association classification (NYHA 165 patients); and 5.7% reduction in NT-proBNP levels (168 points), a HF biomarker.
The safety and efficacy results at 12 months further validate the previously published six-month data.
“These data are consistent and compelling validating that PA pressure-guided therapy improves heart failure outcomes,” said Liviu Klein, section chief of advanced heart failure, mechanical circulatory support, pulmonary hypertension, and heart transplant at the University of California San Francisco (San Francisco, USA) and national principal investigator of the PROACTIVE-HF trial. “The trial results add to the growing understanding of the impact of comprehensive data—seated PA pressure and vital signs—to further improve outcomes, inform remote medical adjustments and directly engage heart failure patients in their own care.
“In the trial, clinicians reduced the PA pressures of congested patients by optimising guideline-directed medical therapy and diuretics to improve heart function. And unique to Cordella, patients have visibility to their health data that helps drive their engagement and compliance.”
Endotronix was acquired by Edwards Lifesciences in July 2024.