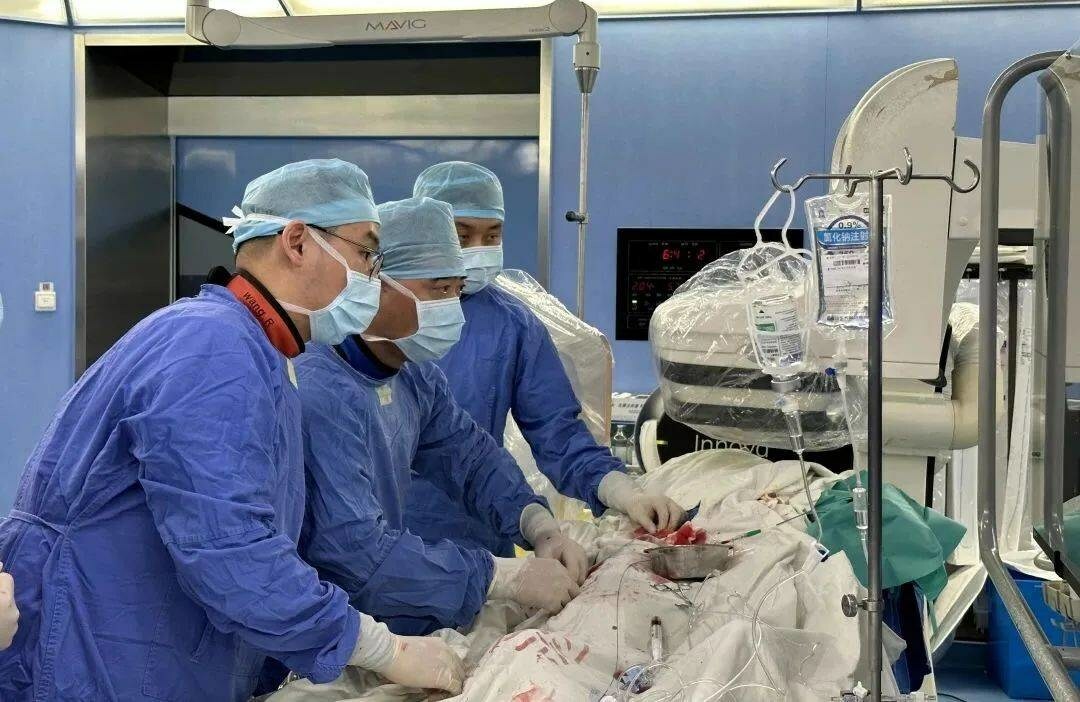
The first-in-man (FIM) use of the NyokAssist (magAssist) percutaneous ventricular assist device (PVAD), supporting a high-risk percutaneous coronary intervention (PCI) procedure, was recently performed by Junbo Ge (Zhongshan Hospital, Shanghai, China) and his team. News of the procedure came shortly after it was announced that the device had received breakthrough designation from the US Food and Drug Administration (FDA).
The patient was a 48-year-old male, diagnosed with coronary artery disease (CAD), heart failure, type II diabetes, and post-PCI before surgery. Preoperative echocardiography showed left ventricular enlargement with decreased left ventricular systolic motion, with a left ventricular ejection fraction (LVEF) of 34%.
Given the patient’’s medical condition and high surgical risk, Ge’s team evaluated the possible haemodynamic disorders during the procedure requiring mechanical circulatory support and decided to perform PCI with the support of NyokAssist.
With the insertion of a 9Fr sheath, NyokAssist was deliver through the aortic arch and valve, providing an average flow of more than 3L/min. The patient’s blood pressure was stable during the procedure. The patient’s recovery culminated in discharge two days after the procedure, magAssist said in a press release.