Medtronic has announced the launch of its Penditure left atrial appendage (LAA) exclusion system in the USA.
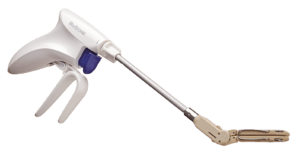
The Penditure LAA Exclusion System is an implantable clip that comes pre-loaded on a single-use delivery system for use in left atrial appendage management (LAAM) during concomitant cardiac surgery procedures.
The Penditure clip is curved to better match the atrial anatomy and was designed without fabric for atraumatic closure and reduced inflammation, the company says in a press release. The Penditure device is the only LAA clip that can be recaptured, repositioned, and redeployed after deployment during a procedure, putting greater control in the hands of surgeons.
Medtronic completed the acquisition of the Penditure device technology from Syntheon LLC in August 2023. The acquisition expands the company’s cardiac surgery product portfolio to include left atrial appendage management. The first cases were performed by Gorav Ailawadi and Basel Ramlawi at the University of Michigan Frankel Cardiovascular Center (Ann Arbor, USA) and Lankenau Heart Institute (Wynnewood, USA), part of Main Line Health, respectively.
“This launch brings innovation to the space, offering a solution that is low profile and can provide more control and visibility than ever before. And while we hope we do not need to use it often, the Penditure clip is recapturable and redeployable should we ever want to reposition an already deployed clip, which is a nice safety feature,” said Ailawadi.
”The Penditure device was effective at completely excluding the LAA at its base, achieving an excellent result surgically and on echo imaging. Given successful initial experience, surgeons can place the device safely and reliably while having the added benefit of repositionability if needed for optimal placement,” said Ramlawi.
The American College of Cardiology (ACC), American Heart Association (AHA), and Society of Thoracic Surgeons (STS) guidelines recommend that patients undergoing a concomitant cardiac surgery procedure and have pre-operative atrial fibrillation (AF) should have their LAA closed. This is because patients with AF experience a five times greater risk of stroke, and most stroke-causing clots originate in the LAA. Closing the LAA may prevent these blood clots from entering the bloodstream.
Medtronic will begin enrolment in the exClusion of the left atrial appendage with PendITure (CLIP-IT) post-market study, in early calendar year 2024. The study will aim to further evaluate the performance and clinical outcomes of the Penditure LAA exclusion system in subjects undergoing concomitant cardiac surgery. CLIP-IT will be a multicentre, single-arm, non-randomised, interventional, post-market study enrolling approximately 150 patients at 25 US sites.
“The strategic addition of the Penditure left atrial appendage exclusion system demonstrates our commitment to investing in cardiac surgeons and their growing needs for managing patients with more complex cardiac disease. The Penditure device reinforces our commitment to innovation and provides an important, new, differentiated LAA management option for cardiac surgeons in the care of their patients,” said Karim Bandali, president of the cardiac surgery business within the cardiovascular portfolio at Medtronic.
The Penditure LAA Exclusion System received a 510k clearance in August 2023 and is commercially available in the USA on a limited basis at this time.










