Medtronic Canada has received a new expanded indication from Health Canada for its Evolut transcatheter aortic valve implantation (TAVI) system. Medtronic’s TAVI platform is currently the only system licensed for both bicuspid aortic valves (intermediate or greater risk), as well as all surgical risk categories in Canada.
Evolut is now indicated in Canada for severe aortic stenosis patients across all risk categories (extreme, high, intermediate and low). With this new indication, over 10,500 people in Canada are potential candidates for this minimally invasive alternative to open-heart surgical valve replacement (SAVR) each year. The expanded indication also allows patients with bicuspid aortic valves at extreme, high and intermediate risk of surgical mortality to also receive the procedure.
“The expansion of TAVI to low surgical risk patients and to those with bicuspid aortic valve disease fills an important gap with a less invasive option than surgical aortic valve replacement,” said Dr. Nicolo Piazza, interventional cardiologist & director of the Structural Heart Program, McGill University Health Centre, Montreal, Canada. “Heart valve teams across Canada are now able to select the type of aortic valve replacement based on patient demographics, anatomical characteristics and patient preference.”
The licensed expanded indication was based on clinical data from the global, prospective, randomised, multi-center Evolut Low Risk Trial, which evaluated three valve generations (CoreValve, Evolut R and Evolut PRO valves) against SAVR in more than 1,400 patients. The data showed TAVI to be an effective option, with shorter hospitals stays and improved 30-day quality-of-life scores compared to SAVR. Additionally, the Evolut system demonstrated superior haemodynamic (blood flow) performance with significantly lower mean aortic valve gradients and larger effective orifice area (EOAs) compared to surgery at one year.
Severe aortic stenosis occurs when the aortic valve becomes diseased (stenotic) and valve leaflets thicken and stiffen, making the heart work harder to pump blood to the rest of the body―negatively impacting daily life. Patients with bicuspid aortic valves have only two functional valve leaflets instead of three leaflets (tricuspid).
“The simultaneous licensing of these indications for the Evolut platform represent major steps toward optimising therapeutic outcomes in these dual populations of aortic valve disease patients,” said Sam Radhakrishnan, interventional cardiologist & medical director of the TAVI Program at the Schulich Heart Centre in Toronto, Toronto, Canada. ”Further, this expanded licence indication makes it possible for health care providers and their patients to now consider minimally invasive therapeutic options in the majority of the aortic stenosis cases encountered.”
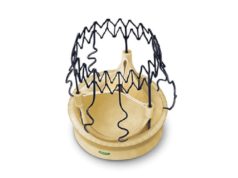
The Evolut TAVI System valve is engineered with a self-expanding nitinol frame that conforms the replacement valve to the native annulus with consistent radial force and includes an external tissue wrap that increases surface area contact with native anatomy for enhanced valve sealing.
“We are proud to further build on our patient portfolio for the Evolut TAVI system so that more Canadians can benefit from this vital, life-saving procedure,” said Mitch Leschuk, senior director of the Cardiovascular portfolio at Medtronic Canada.