Laplace Interventional, a medical device company developing a transcatheter tricuspid valve technology, has completed its first close on its Series B financing. The financing round was co-led by ShangBay Capital along with Features Capital, including participation from Engage Venture Partners, JWC Ventures as well as a global strategic investor.
The US$12.9M Series B financing will fund Laplace Interventional towards achieving its early clinical feasibility milestones. The company also announced that Jenny Barba, co-founder and managing partner at Features Capital, joined its board of directors and Todd Mortier, a medtech entrepreneur had previously joined its board of directors.
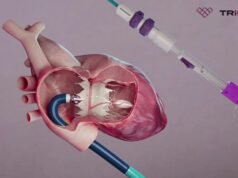
Laplace Interventional’s device is designed to offer an increase in life expectancy and an improvement to the quality of life to patients diagnosed with tricuspid regurgitation (TR). The company is developing a prosthetic valve that will be delivered through through patient vasculature not requiring an open-heart surgery and thereby reducing complications in patients.
“Our team at Laplace has demonstrated feasibility of our technology in a preclinical setting and we are well positioned to execute our clinical feasibility evaluation in the near future,” said Ramji Iyer, founder and CEO of Laplace Interventional. “We are grateful for the validation of our progress from our new and existing investors. We remain laser focused on our mission of improving the standard of care for patients suffering from tricuspid regurgitation.”
“The Laplace team has made tremendous progress in a very short amount of time. ShangBay is thrilled to co-lead this Series B round to be used towards ultimately making their technology useful in improving the lives of patients worldwide suffering from TR,” said William Dai, founding managing partner at ShangBay Capital.
“Laplace has designed an ingenious medical technology and is positioned to solve one of the largest unmet clinical needs remaining in structural heart disease. Tricuspid valve patients and their physicians deserve an advanced world-class solution. Features is honoured to support the remarkable Laplace team in pursuit of their goals,” said Jenny Barba, managing partner of Features Capital.”This investment very much aligns with our focus on supporting entrepreneurs to improve clinical outcomes, increase health access, and reduce costs through novel and differentiated MedTech innovation.”
Laplace Interventional’s device is in the early stage of development phase and is not approved or cleared by the US Food and Drug Administration (FDA) or any other regulatory body in any region of the world.