Data from the National Cardiovascular Data Registry (NCDR) Left Atrial Appendage Occlusion (LAAO) Registry, which enrolled more than 38,000 patients implanted percutaneously with the Watchman device (Boston Scientific), reveal a high implant success rate and a low rate of in-hospital complications. The results offer insights into how real-world implantation of the device compares with findings from premarket clinical trials.
James V Freeman (Yale University School of Medicine, New Haven, USA) presented the analysis at the American College of Cardiology/World Congress of Cardiology’s virtual scientific sessions (ACC.20/WCC Virtual). The results were simultaneously published by the Journal of the American College of Cardiology (JACC).
Freeman and colleagues report as background that while LAAO to prevent stroke in patients with atrial fibrillation (AF) has been evaluated in two randomised trials, post-approval clinical data are limited. The LAAO registry included data from nearly 500 hospitals and more than 1,300 physicians in the USA.
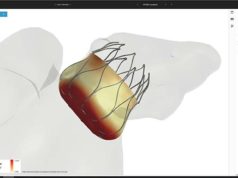
Watchman is a device that blocks the left atrial appendage to prevent blood clots from forming there, where they can exit the heart and cause strokes. The US Food and Drug Administration (FDA) approved it in 2015 for reducing the risk of stroke in patients with AF, a press release from the ACC states. Patients are eligible for a Watchman device if they have AF that is not caused by valve problems, face a moderate or high risk of stroke and cannot take anticoagulant medications.
The NCDR LAAO registry is a national programme for post-market Watchman surveillance, to which, as of April 2016, US hospitals were required to report all Watchman procedures performed in order to qualify for Medicare reimbursement. The registry was established to provide a snapshot of the procedures involving the device during its first three years of use (2016–2018) in the USA and describe patient, hospital and physician characteristics and in-hospital adverse event rates.
In the ACC.20/WCC Virtual session, Freeman described the LAAO Registry structure and governance, the outcome adjudication processes, and the data quality and collection processes. He also characterised the patient population, performing hospitals, and in-hospital adverse event rates.
Importantly, patients in the registry were generally older with more comorbidities than those enrolled in the pivotal trials; however, major in-hospital adverse event rates were lower than reported in those trials. Freeman and colleagues write that major in-hospital adverse events occurred in 2.16% of patients with the most common complications being pericardial effusion requiring intervention (1.39%) and major bleeding (1.25%), while stroke (0.17%) and death (0.19%) were rare.
“There is clearly a lot of enthusiasm in the post-market setting in the US to undertake this procedure as an alternative to blood thinners for these patients, who can be stuck between a rock and a hard place because they are at risk for atrial fibrillation-related stroke but have had prior bleeding or other problems with blood thinners,” said Freeman, a cardiac electrophysiologist and associate professor of medicine at Yale School of Medicine and the study’s lead author in a press release from the ACC. “Patients getting this procedure in the real world are generally older and sicker than the patients who were in clinical trials, and it is reassuring to see that the procedural safety profile looks good.”

Key datapoints from the registry
- The mean patient age was 76.1±8.1 years, the mean CHA2DS2-VASC score was 4.6±1.5, and the mean HAS-BLED score was 3.0±1.1.
- The median annual number of LAAO procedures performed for hospitals was 30 (interquartile [IQR] range 26) and for physicians was 12 (IQR 12).
- Procedures were cancelled or aborted in 7% of cases; among cases in which a device was deployed, 98.1% were implanted with <5mm leak.
In an accompanying editorial in the JACC, Dhanunjaya Lakkireddy (University of Missouri – Columbia, Kansas, USA) and Mohit Turagam (Icahn School of Medicine at Mount Sinai, New York, USA) write: “It is actually very encouraging to see that the rate of complications did not peak, as is typically anticipated in post-market release studies. Quite contrary to the Manufacturer and User Facility Device Experience (MAUDE) database studies, the actual NCDR LAAO registry shows relatively low rates of complications despite device implantation being performed by a large number of novice operators and in institutions that were not part of the original randomised controlled trials. This is purely a reflection of the stringent criteria laid down by CMS to improve the post-approval safety profile of these type of interventions. On many fronts, this is a success story”.
Data limitations
Outlining some of the limitations of the data, Lakkireddy and Turagam note: “First, this is an observational, non-randomised analysis of periprocedural outcomes of the Watchman LAAO device from a registry with no control group and did not mandate consecutive enrolment of patients. Great caution is required while interpreting these results due to risks of unmeasured confounders. Second, although the reported adverse events were adjudicated by a validated computer-based algorithm, the LAAO Registry relies on site-reported data with no other system-based mechanisms to identify any under-reported or misclassified events. This raises the issue of under-reporting and underestimation of adverse events.”