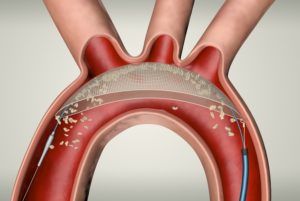
Keystone Heart has announced completion of the first worldwide commercial case using the TriGUARD 3 cerebral embolic protection (CEP) device. Pieter Stella, assistant professor, Medical Department of Cardiology, at UMC Utrecht, Utrecht, the Netherlands, performed the transcatheter aortic valve implantation (TAVI) procedure using the TriGUARD 3 device, which is designed to minimise the risk of cerebral damage during transcatheter heart procedures.
“Although we have seen improvement in mortality and morbidity rates during TAVI procedures, the risk of periprocedural stroke has not changed,” commented Stella. “As a physician, I am driven to do anything possible to prevent this rare but terrible complication. I am pleased we can now routinely use the TriGUARD 3 CEP device in all of our TAVI cases to protect patients from stroke, delirium, and neurocognitive decline.”
The CE marked TriGUARD 3 CEP device is designed to cover and protect all three major cerebral aortic arch vessels. The Nitinol frame with dome-shaped mesh deflector is delivered transfemorally and designed to “self-position” in the aortic arch. This design allows the TriGUARD 3 CEP device to conform to a variety of patient anatomies, Keystone Heart said in a press release.
“Our team is thrilled to commercially launch the only CE marked device designed to cover and protect all three cerebral vessels,” stated Chris Richardson, Keystone Heart president and CEO. “Our primary objective is to ensure a controlled launch that provides physicians the training and tools necessary to safely and consistently treat all patients undergoing trans-catheter heart procedures with the TriGUARD 3 CEP device.”
Richardson further commented: “As TAVI procedures continue to expand into treating lower-risk patient groups, the need to protect patients’ brains will also grow. Strokes and minor cognitive events can have devastating outcomes for patients and their families; Keystone Heart is committed to helping physicians minimize those occurrences.”
Keystone Heart began commercialisation of the TriGUARD 3 CEP device in TAVI centres of excellence throughout Europe in July 2020.