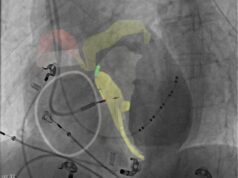
Jupiter Endovascular has exited stealth mode with a US$21 million round of financing, with the funding will be used to support the company’s upcoming pivotal trial for pulmonary embolism and development of additional clinical applications for its Endoportal Control technology.
Jupiter Endovascular has exited stealth mode with a US$21 million round of financing, with the funding will be used to support the company’s upcoming pivotal trial for pulmonary embolism and development of additional clinical applications for its Endoportal Control technology.
“For decades, the field of catheter-based therapies has been limited by the technological constraints of catheters that lose stability and control within the anatomy,” said Kate Garrett, managing partner at Sonder Capital. “Jupiter Endovascular has developed a revolutionary approach with the potential to overcome these constraints by providing clinicians the precision and control of a surgical approach while maintaining the minimally invasive profile of an endovascular procedure. This novel approach may address a significant unmet clinical need in patients with disease residing in complex cardiovascular anatomies, such as the pulmonary arteries.”
The company has appointed Carl J St Bernard as chief executive officer. St Bernard joins Jupiter from Alta Biomaterials, where he held the role of president and CEO. He has over 30 years of experience in the life sciences arena and has held senior executive positions at CeloNova BioSciences, Tryton Medical, Johnson & Johnson Vision, LifeCell, Cordis, and GE Healthcare. He is also an AdvaMed board director.
“I am very excited to join the Jupiter Endovascular team. The Endoportal Control technology, which leverages Jupiter’s unique and exclusive intellectual property to create a catheter-based device that flexibly navigates through a patient’s vasculature before fixing into a stable position, is designed to give interventionalists the confidence and support they need to deliver a prescribed cardiovascular therapy. This represents a generational advance in technology with the potential to revolutionise the treatment of millions of patients worldwide,” said St Bernard. “We are thrilled to partner with Sonder Capital and our other highly valued investors as we bring this innovation to the clinic and create the foundation for our entry into the pulmonary embolism market and future clinical areas where we see a compelling opportunity for Endoportal Control to improve the lives and well-being of patients.”