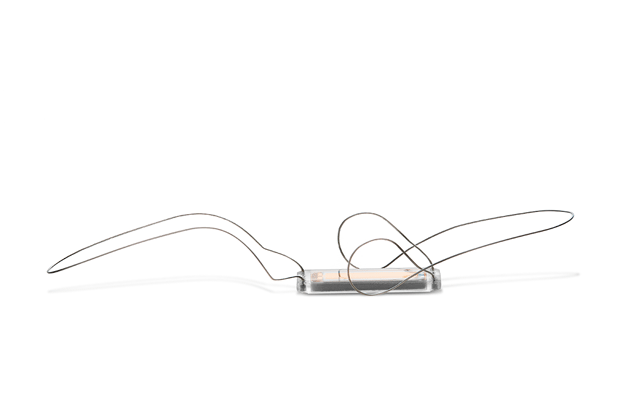
Endotronix has announced the 100th global implant of the Cordella pulmonary artery pressure sensor. The implant was performed by Faisal Sharif of Bon Secours Hospital and director of Cardiovascular Research at NUIG, both in Galway, Ireland.
The sensor provides key heart pressure readings as part of the Cordella heart failure system to empower clinicians to proactively adjust therapy and medications remotely without the need for office visits, Endotronix said in a press release.
The milestone included patient enrolment across the company’s three clinical trials: SIRONA, SIRONA II, and PROACTIVE-HF. The SIRONA II and PROACTIVE-HF trials, which are currently enrolling, will support regulatory submission for CE mark and Pre-Market Approval (PMA) of the pulmonary artery (PA) sensor and lead the company towards global commercialisation.
“PA pressure-guided heart failure management is fast becoming the standard of care in NYHA Class III patients,” stated Liviu Klein (University of California San Francisco, San Francisco, USA), national principal investigator of the PROACTIVE-HF trial. “As clinicians look to better manage heart failure, our goal is to improve quality of life for patients while keeping them out of the hospital, which is particularly important in today’s times. With the comprehensive clinical data provided by the Cordella system with the PA sensor, my team can remotely titrate medications to deliver guideline directed therapy and ultimately, improve patient outcomes.”
The Cordella System enables scalable remote heart failure management and aims to increase guideline directed medical therapy (GDMT) adherence and provide early detection of worsening heart failure. The platform consists of a comprehensive patient management system that securely collects non-invasive daily health data, coupled with a seamlessly integrated, next-generation implantable PA pressure sensor. Together, they deliver the necessary information for clinicians to proactively titrate medications and improve patient care between office visits while supporting reimbursement for care delivery activities.
“Our clinical partners are amazing. We appreciate their tremendous effort in achieving this significant milestone and for their tireless dedication to improving patient care,” added Harry Rowland, co-founder and chief executive officer of Endotronix. “Our team continues to focus on supporting our clinical sites and accelerating trial enrolment as we look to build momentum in preparation for a successful global commercialisation of the Cordella Sensor.”










