 MedAlliance has announced enrolment of over 1,660 patients in its SELUTION DeNovo coronary randomised study. Recruitment is now halfway towards a planned 3,326 patients.
MedAlliance has announced enrolment of over 1,660 patients in its SELUTION DeNovo coronary randomised study. Recruitment is now halfway towards a planned 3,326 patients.
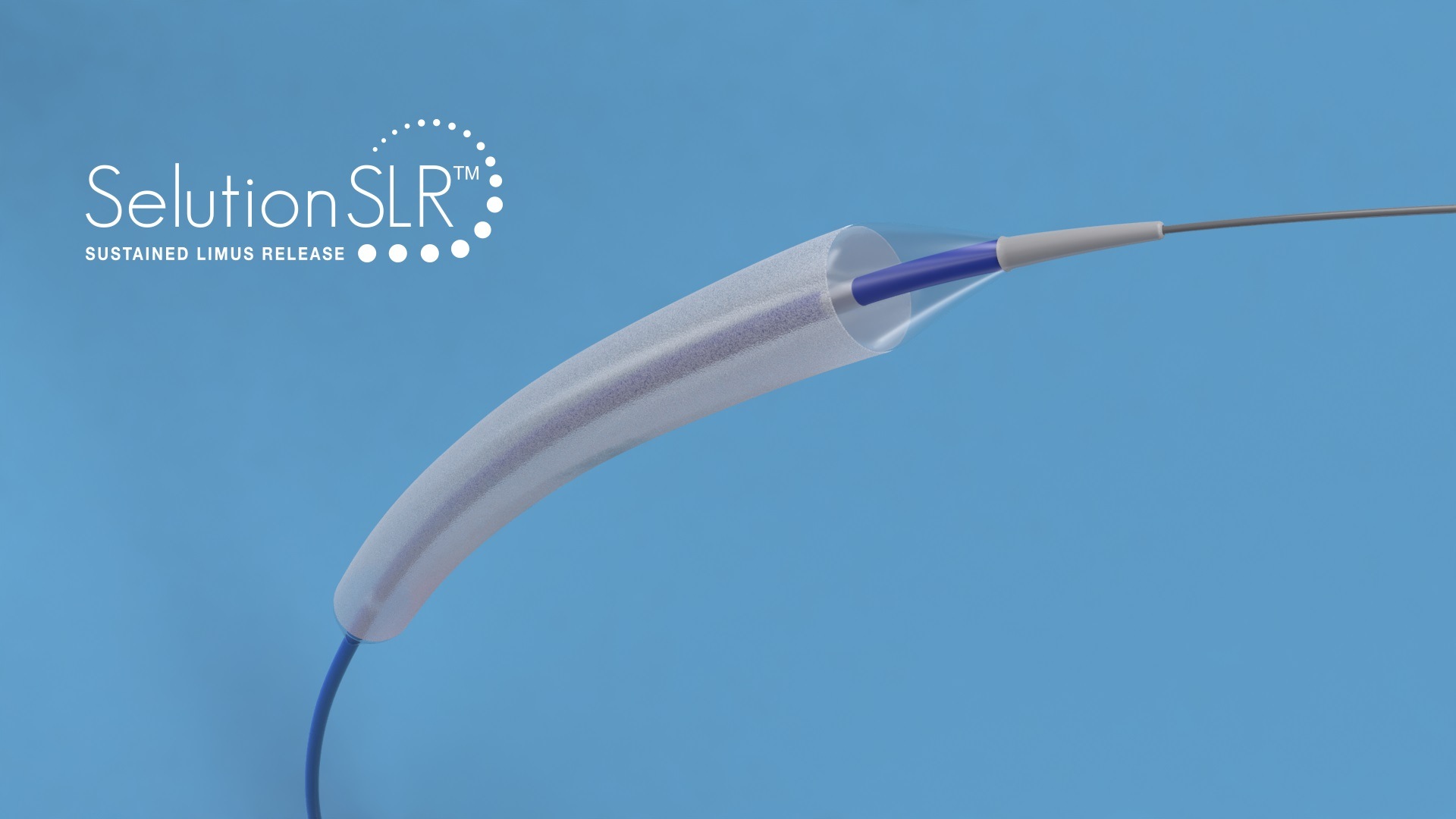
SELUTION DeNovo compares the treatment strategy using a novel sirolimus drug-eluting balloon—Selution SLR—versus any limus drug-eluting stent [DES].
MedAlliance describes SELUTION DeNovo as the largest DEB study ever initiated, involving up to 70 participating sites across 15 countries. Patients are randomised before any vessel preparation to reflect current medical practice and to reduce bias. The objectives of the study are to demonstrate non-inferiority at both one and five years, and superiority for target vessel failure (TVF) at five years.
Selution SLR consists of an angioplasty balloon coated with micro-reservoirs containing a mixture of biodegradable polymer and the anti-restenotic drug sirolimus. The micro reservoirs provide controlled and sustained release of the drug for over 90 days, similar to a DES, but without leaving behind a metal stent, which has been associated with a complication rate of 2% annually.
“This is a major milestone for the SELUTION DeNovo trial, as it is now the largest DEB study ever conducted”, said co-principal investigator Christian Spaulding (European Hospital Georges Pompidou, Assistance Publique Hôpitaux de Paris and Paris Cité University, Paris, France). “The study is performed in a true all-comers population and is not just looking at small vessel artery disease. It is encouraging that the Drug and Safety Monitoring Board has no concerns, voting unanimously for the trial to continue as planned, as there are no worrying protocol deviations and no significant differences between the groups. We see investigators being comfortable using this novel approach and expect the enrolment to be completed within the next 12 months.”
“This trial has the potential to change medical practice, not only in Europe, but also in the USA, China and Japan, benefitting patients around the globe”, added Jeffrey B Jump, MedAlliance chairman and CEO. “We are currently enrolling US patients in our IDE coronary ISR, peripheral BTK and SFA studies. The coronary SELUTION4DeNovo IDE trial is due for enrolling its first US patient at the beginning of Q4 2023”.
Selution SLR was awarded CE mark approval for the treatment of coronary artery disease in May 2020. MedAlliance was the first drug-eluting balloon company to receive US Food and Drug Administration (FDA) breakthrough designation status, and received coronary in-stent restenosis (ISR) investigational device exemption approval in October 2022 and de novo coronary artery lesions approval on 6 January 2023. This will complement the substantial experience that the company has gained with the SELUTION DeNovo trial in Europe.
Selution SLR is commercially available in Europe, Asia, the Middle East, and the Americas (outside USA) and most other countries where the CE mark is recognised. Over 40,000 units have been used for patient treatments in routine clinical practice or as part of coronary clinical trials.













