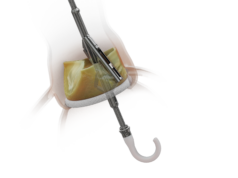
Pi-Cardia has announced the first commercial procedures with its ShortCut dedicated leaflet modification device, enabling valve-in-valve (ViV) transcatheter aortic valve implantation (TAVI) procedures in patients at risk of coronary obstruction. This follows the news of ShortCut’s de novo market clearance granted by the US Food and Drug Administration (FDA) in September 2024.
The initial ShortCut procedures were successfully performed at various institutions including the Gagnon Cardiovascular Institute in Morristown (USA) by Philippe Généreux and Gennaro Giustino, at Cedars-Sinai Medical Center (Los Angeles, USA) by Raj Makkar and Wen Cheng, and at Piedmont Heart Institute (Atlanta, USA) by Vinod H Thourani and Pradeep Yadav.
“We are honoured to have performed the first commercial ShortCut procedure and to be at the forefront of the innovative field of leaflet modification,” says Généreux. “In the past, treating this challenging patient population was possible mainly with surgical approaches or complex techniques. With ShortCut, we safely split both the RCC [right coronary cusp] and LCC [left coronary cusp] leaflets in a simple, predictable, and controlled manner, enabling safe placement of a TAVI.”
“The ShortCut device is a true game changer, addressing one of the most significant challenges in structural heart,” says Makkar. “It will play a fundamental role in the lifetime management of aortic stenosis patients who may require multiple valve interventions. Given its quick and intuitive nature and seamless integration into the standard TAVI workflow, I am confident this tool will become highly adoptable across TAVI centres.”
TAVI has become the preferred treatment for aortic stenosis, recently surpassing surgery even in patients younger than 65. As bioprosthetic valves degenerate over time, these patients will at some point likely need a valve-in-valve procedure to be performed.
“ShortCut is transforming the way we approach patients with aortic stenosis,” say Yadav and Thourani. “For intermediate- and high-risk patients who would have otherwise undergone surgery, it provides a reliable and predictable transcatheter option to prevent coronary obstruction. Thinking beyond the valve, we are now considering the necessary steps before implantation, as this will directly impact future treatment options for these patients.”
Recently published models predict that by 2035, more than 40,000 valve-in-valve procedures will be performed annually in the USA, representing more than 15% of all TAVI procedures.
Future planned indication expansion into native and bicuspid valves may mean that around 30% of future TAVI cases will require leaflet modification in order to be performed safely and obtain optimal results for patients, Pi-Cardia says in a press release.
“We are incredibly proud of this significant milestone and are excited to pioneer the leaflet modification market,” said Erez Golan, chief executive officer of Pi-Cardia. “The first week of US launch demonstrated strong demand across the country for ShortCut, with several leading hospitals treating patients successfully, and many other recognised institutes, including Tucson Medical Center, Kaiser San Francisco, Los Robles Regional Medical Center, University of Michigan, and Sentara Norfolk General Hospital, expected to join in the coming weeks. We continue to build our highly skilled field team and further increase manufacturing capacity as we partner closely with physicians and hospital systems to ensure they have access to ShortCut.”