 The first patients have been enrolled in the FAST III trial at the Erasmus University Medical Center in Rotterdam, The Netherlands. This marks the beginning of a multicentre, randomised controlled, open-label study in which the outcome of therapy for coronary artery disease is being investigated after fractional flow reserve (FFR) has been determined using different methods. The principal Investigator in the FAST III study is Joost Daemen, cardiologist in the Thoraxcenter at the Erasmus University Medical Center.
The first patients have been enrolled in the FAST III trial at the Erasmus University Medical Center in Rotterdam, The Netherlands. This marks the beginning of a multicentre, randomised controlled, open-label study in which the outcome of therapy for coronary artery disease is being investigated after fractional flow reserve (FFR) has been determined using different methods. The principal Investigator in the FAST III study is Joost Daemen, cardiologist in the Thoraxcenter at the Erasmus University Medical Center.
FFR evaluates the haemodynamic relevance of a coronary stenosis by determining the ratio between the maximum achievable blood flow in the diseased segment and the theoretical maximum flow under normal conditions.
In the standard procedure for determining FFR, a guide wire is inserted into the coronary artery. It is equipped with an electronic pressure sensor to measure the pressure drop before and after the stenosis. In this procedure, the patients is given an infusion of adenosine to simulate a stress situation. This can lead to unpleasant conditions, including restlessness, shortness of breath, and chest pain.
The FAST III study compares the impact of percutaneous coronary intervention (PCI) guided by CAAS vFFR (Cardiovascular Angiographic Analysis Systems for vessel Fractional Flow Reserve), a software from Pie Medical Imaging, with the outcome of PCI guided by the conventional, invasive, pressure wire-based method. FAST III is subtitled “Fractional Flow Reserve or 3D-Quantitative-Coronary-Angiography Based Vessel-FFR Guided Revascularization”.
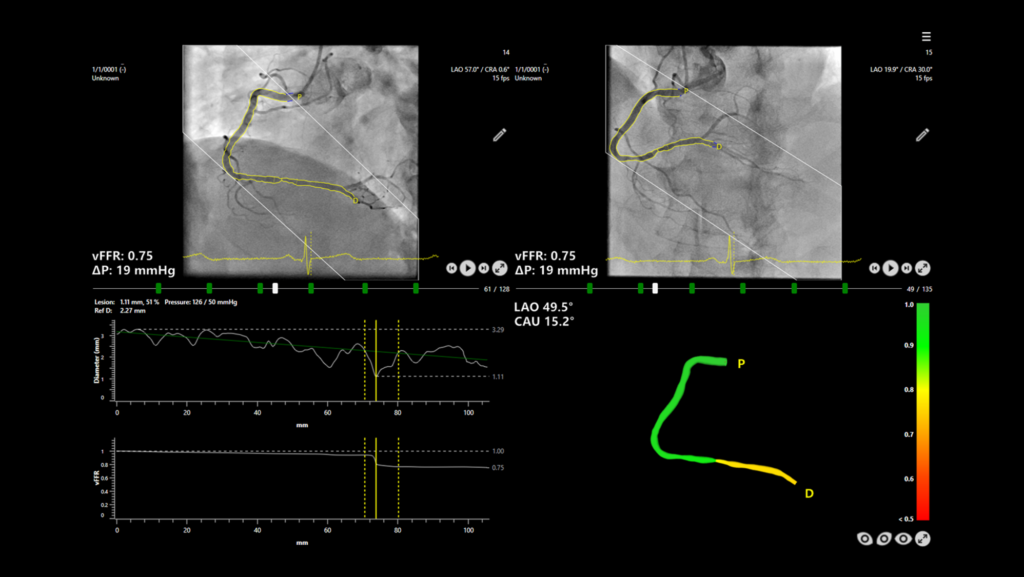
CAAS vFFR is an innovative technology that enables a less invasive physiological evaluation of coronary lesions, Siemens Healthineers said in a press release. Pressure drop and the vFFR (vessel fractional flow reserve) value are determined on the basis of two angiographic images, which eliminates the need for an invasive pressure wire and hyperemic agent.
In the FAST I1, FAST EXTEND2, and FAST II3 studies, CAAS vFFR was shown to be an easy-to-use tool for assessing coronary physiology that provides high diagnostic accuracy when assessing the severity of a lesion, the press release adds.
“In the retrospective FAST I and FAST EXTEND trials we recently validated the vFFR software and demonstrated that vFFR had a high linear correlation to invasive pressure wire based FFR and a high diagnostic accuracy alongside with a very low interobserver variability. These finding were then subsequently confirmed in the prospective multicentre FAST II trial with the help of an independent blinded core lab,” Daemen explained.
FAST III will help determine if the therapeutic outcomes for the study participants with intermediate coronary artery stenosis are comparable, whether the decision for coronary revascularisation was made based on FFR with CAAS vFFR or with the pressure wire procedure.
“We believe that FAST III trial will be an important next step in demonstrating the clinical value of vFFR in guiding the physiological importance of intermediate coronary artery lesions in patients with either stable or unstable coronary artery disease. Non-inferiority of vFFR as compared to a FFR-guided treatment strategy could confirm another important step forward in simplifying physiological lesion assessment by abandoning the need of a pressure wire or micro-catheter and the use of an hyperemic agent with known side effects to the patients,” commented Daemen.
A total of 2,228 patients in up to 35 hospitals in seven European countries will be enrolled in the study; randomised in a one-to-one process to either vFFR- or FFR-guided approach. The leading clinical centers involved include, but are not limited to: Erasmus University Medical Center (The Netherlands), John Radcliffe Hospital Oxford (UK), Mater Private Hospital Dublin (Ireland), University Hospital of Verona (Italy), University Hospital Valladolid (Spain), Charité Berlin (Germany), and University Hospital Lille (France).
The European Cardiovascular Research Institute (ECRI) in Rotterdam, The Netherlands, is the sponsor of the investigator-initiated FAST III trial. FAST III is funded by Pie Medical Imaging and Siemens Healthineers, who joined forces to strengthen the body of evidence for the use of angio-derived FFR in the pursuit of a faster and less stressful diagnosis along with the potential for a lower cost of care.










