The first patient in the APOLLO trial has undergone transcatheter mitral valve implantation (TMVI) with the Intrepid device (Medtronic). The study—the first-ever pivotal trial of TMVI therapy—will evaluate the safety and efficacy of the Intrepid device in up to 1,200 patients with severe, symptomatic mitral valve regurgitation. The Intrepid device recently received investigational device exemption (IDE) approval from the US FDA.
The APOLLO trial design consists of two cohorts and will be conducted at up to 60 sites to evaluate two distinct patient populations. The primary endpoint in both cohorts of the trial is a composite endpoint rate of all-cause mortality, all-stroke, reoperation (or reintervention) and cardiovascular hospitalisation at one year with secondary endpoints that measure quality of life and valve performance in patients with severe symptomatic mitral regurgitation.
The randomised cohort will enrol up to 650 patients who are candidates for conventional open-heart mitral valve replacement surgery and not eligible for mitral repair. These patients will be evenly randomised to receive either the Intrepid TMVI system or conventional mitral valve surgery. The primary endpoint is designed to demonstrate the Intrepid TMVI system is statistically non-inferior to conventional surgery at one year.
The single arm cohort will enrol up to 550 patients who are considered too high a risk for conventional open-heart mitral valve surgery, as determined by a multidisciplinary heart team, and will be assigned to undergo the TMVI procedure with the Intrepid system. The primary endpoint of this cohort is designed to demonstrate statistical non-inferiority to a performance goal at one year.
The first patient in APOLLO was implanted by the team at Aurora St. Luke’s Medical Center (Milwaukee, USA).
David H Adams (Mount Sinai Health System, New York , USA), national co-principal investigator of the APOLLO trial, comments: “This is the beginning of an important journey to establish a truly less invasive approach to treat severe mitral valve regurgitation in patients who are appropriate candidates for mitral valve replacement with a transcatheter technology that eliminates the need for open-heart surgery.”

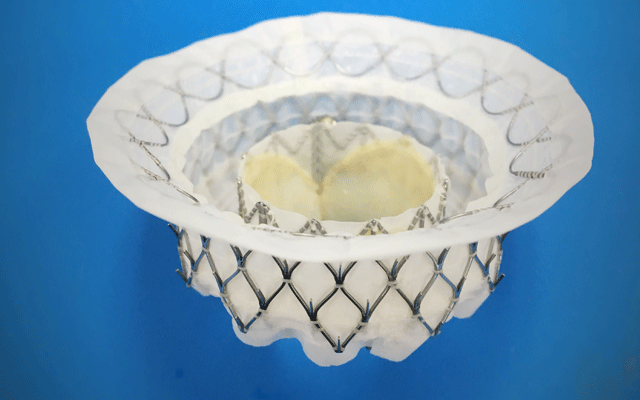
The Intrepid TMVI system, according to a press release, integrates self-expanding, dual-stent technology with a replacement tissue heart valve to facilitate a catheter-based implantation. The valve is compressed inside a hollow delivery catheter and is inserted between the ribs to enter the heart. The new replacement valve is expanded directly into the malfunctioning mitral valve. The outer stent frame is designed to attach and conform to the native valve without the need for additional sutures, tethers, or anchors to secure the prosthesis. The inner stent houses the valve, which is made from bovine tissue and is intended to maintain blood flow.
“The Intrepid system features a truly innovative dual-stent design and the trial will investigate its safety and efficacy in addressing severe symptomatic mitral regurgitation replacing the need for an open-heart procedure. We look forward to working with the APOLLO trial clinical sites in the USA and around the world,” says Martin Leon (Interventional Vascular Therapy, Columbia University Medical Center/New York-Presbyterian Hospital, New York, USA). Leon is the national co-principal investigator of the APOLLO trial.













