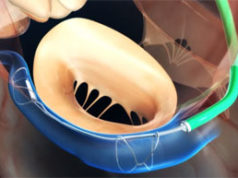
Cardiac Dimensions has announced the company has randomised its first patient in the CARILLON pivotal trial, which is evaluating the Carillon mitral contour system for the treatment of functional mitral regurgitation associated with heart failure as compared to a randomised control group treated with optimal medical management according to established heart failure guidelines
The multicentre, double-blinded, randomized controlled trial is expected to randomise 450 patients at up to 75 centres in North America and Europe. The trial has primary safety and efficacy endpoints at 12 months and will follow the randomised patients out to five years to document long-term safety and clinical status. The CARILLON trial also includes a crossover feature that allows patients originally randomised to the control group to receive the Carillon System after their one-year follow-up visit.
Samir Kapadia (Heart & Vascular Institute, Cleveland Clinic, Cleveland, USA), one of the principal investigators of the CARILLON trial, says: “Current treatments to improve the quality of life for patients with functional mitral regurgitation fall short and many heart failure patients are too frail for open-heart surgery. Transcatheter treatments have been shown to be safe and effective in the treatment of valve diseases, but there is no minimally invasive intervention yet approved in the USA. for these patients. Today, most functional mitral regurgitation patients are treated with medical therapy only for decreasing symptoms but this does not address the underlying anatomical problem leading to mitral regurgitation. The CARILLON trial should provide us a better understanding of the benefits of the Carillon System as an option for heart failure patients with functional mitral regurgitation.”
A press release explains that the Carillon system addresses the underlying mechanical problem of functional mitral regurgitation with a catheter-based alternative to medications and invasive surgery. Tomasz Siminiak (Poznan University of Medical Sciences, Poznań, Poland) randomised the first patient in the CARILLON trial. He says: “We are very excited to participate in this landmark trial to study the Carillon system for patients with functional mitral regurgitation. These patients need access to less invasive treatment options, and to be able to contribute to advancements that have the potential to slow the progression of this chronic disease, is very important to us.”
Gregory D Casciaro, president and CEO of Cardiac Dimensions, comments: “The initiation of the CARILLON Trial is the pinnacle of our clinical programme, which includes positive data from three previous clinical trials, and is the final step on our path to bringing the Carillon System to patients in the United States. Our vision is to have a significant impact on the treatment of patients with functional mitral regurgitation, offering physicians a safe and easy-to-use option that can treat a broader range of patients with the goal of slowing disease progression and preventing worsening quality of life.”