The first stage of the first human implantation of the RoseDoc (TruLeaf Medical) docking system for transcatheter replacement of the tricuspid valve has been completed as part of a trial of the device.
The procedure was successfully performed on 5 September in India using the compassionate care pathway, involving two patients suffering from severe refractory congestive heart failure due to leaking tricuspid valve who had no alternative therapeutic options.
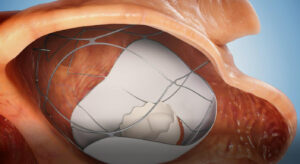
The second phase of percutaneously implanting the implantation of the valve itself developed by the company withing the docking device, is expected to be performed in the coming months according to the trial protocol.
TruLeaf’s technology enables the implantation to be performed in two stages. First, a docking station is implanted, and subsequently the heart valve is implanted.
“This trial is not only an important step for TruLeaf Medical, but also a huge step for the entire medical community and a ray of hope for millions of patients,” says Oz Shapira, chief executive officer of Allmed Solutions Group, the owner of TruLeaf Medical. “As a heart surgeon, I deeply understand the absolute necessity of transitioning from a high-risk invasive and complex open-heart surgery to a simple, smart, safe and effective catheter-based solution. TruLeaf Medical RoseDoc platform has the potential to offer an effective treatment to millions of patients with valvular heart disease who hare deemed to be too high risk for surgery and are unsuitable to currently available catheter-based techniques.”
TruLeaf Medical was founded in 2017 by Israeli entrepreneurs Benjamin Spencer, Nathaniel Benisho and Uri Rosenstein. Spencer and Benisho were involved in the development of the first ever transcatheter aortic bioprosthesis, initially within the Israeli company PVT, that was later acquired by Edwards Lifesciences.
TruLeaf Medical continues with patient recruitment for the first-in-human trial in India and Uzbekistan, and plans to expand the participating sites in additional countries. The first human implantation represents a critical milestone and will accelerate the process of regulatory approval making this innovative solution available to numerous patients worldwide.










