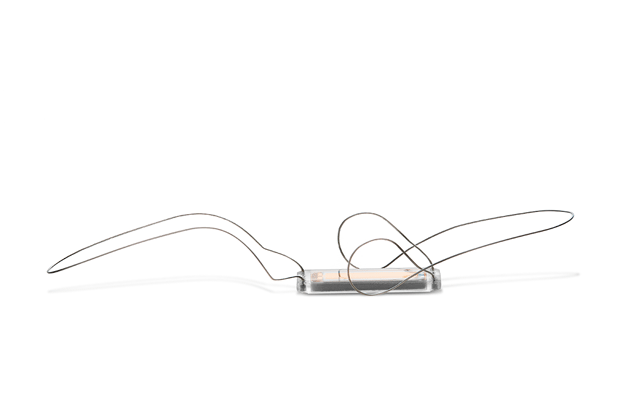
Endotronix has announced successful first-in-human implantation of the Cordella pulmonary artery pressure sensor and the initiation of the SIRONA first-in-human (FIH) clinical trial. Wilfried Mullens (Ziekenhuis Oost-Limburg and University Hasselt) and Matthias Dupont (Ziekenhuis Oost-Limburg), both co-investigators of the SIRONA trial, performed the first implant at the Hospital Ziekenhuis Oost-Limburg in Genk, Belgium.
A press release reports that the the SIRONA FIH trial is designed to evaluate the safety and performance of the Cordella pulmonary artery pressure sensor in the treatment of advanced heart failure patients who remain unstable despite standard of care medical management. The wireless Cordella device, the implantable component of the Cordella heart failure system, is designed to provide clinicians with pulmonary artery pressure readings that they can then interpret to guide therapy and provide proactive heart failure management.
Clinical studies have shown that regular pulmonary artery pressure monitoring can provide earlier detection than other modalities of worsening heart failure, decrease heart failure-related hospitalisations by 37%, and reduce mortality by 57% for patients with heart failure with a reduced ejection fraction.
Mullens says: “We were extremely pleased with the Cordella pulmonary artery pressure sensor implantation procedure experience and initial wireless pulmonary artery pressure measurements. Tecent clinical data points to the value of regular pulmonary artery pressure monitoring as an early indication of worsening of a patient’s heart failure condition. And coupling the sensor with Endotronix’s patient management platform for heart failure has the potential to improve the way this patient population is managed.”
The SIRONA trial will enrol up to 10 patients at two European sites. The primary safety endpoint is freedom from adverse events related to use of the system through 30-days post sensor implantation. Accuracy of the Cordella pulmonary artery pressure sensor readings as compared to a fluid-filled pulmonary artery pressure measurement taken by right heart catheterisation at 90-days is the primary efficacy endpoint.
“Implanting our first patient in the SIRONA trial is a significant achievement that moves us closer to our goal of providing comprehensive, proactive heart failure management. We look forward to furthering the field of heart failure management by combining best-in-class sensing technology with best-in-class disease management solutions,” says Harry Rowland, chief executive officer and co-founder of Endotronix.
According to the press release, the Cordella heart failure system is a comprehensive, disease management system that enables on-going therapeutic interventions to improve patient quality of life and decrease heart failure readmissions. The system is designed to collect and securely transmit biometric and relevant clinical data to the heart failure clinician, aiming to create accurate snapshots of the patient’s health status over time.









