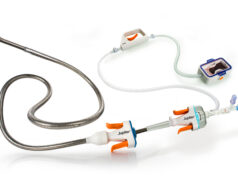
Reprieve Cardiovascular has announced the completion of enrolment in the FASTR trial, comparing the effectiveness of decongestive therapy administered by the Reprieve system with optimal diuretic therapy (ODT) in patients diagnosed with acute decompensated heart failure (ADHF).
The primary objective of the study is to determine whether the Reprieve system can more efficiently decongest ADHF patients compared to standard-of-care therapy.
“For decades, managing fluid removal in patients with ADHF has presented a significant challenge, with many failed trials of new drugs or devices,” said James Udelson (Tufts Medical Center, Boston, USA), principal investigator of the FASTR trial. “Diuretics, which have been the only widely used therapeutic option, are difficult to administer precisely without real-time information on patient response, making it challenging to balance rapid fluid removal with the risk of kidney injury, and thus are rarely used optimally. In addition, these patients are often faced with lengthy hospital stays, incomplete decongestion at discharge and frequent readmissions due to fluid volume overload. The Reprieve system is a promising heart failure management solution that can be easily implemented into existing clinical workflows, while also elevating the current level of care. As a result, this may help ease the workload burden for clinicians treating heart failure patients.”
Intended to personalise decongestion management and safely, quickly, and completely remove excess fluid to improve patient outcomes and prevent hospital readmissions, the Reprieve system works with the kidneys to remove fluid through precise administration of diuretics, rapidly finding the optimal dose while ensuring they receive an adequate amount of sodium to support optimal kidney function.
The Reprieve system combines real-time physiological monitoring with automated recommendations to escalate or end therapy, enabling physicians to tailor treatment to each patient’s specific needs during therapy. This approach aims to safely accelerate fluid removal and improve patient outcomes while reducing the likelihood of kidney injury and hospital readmissions for patients with ADHF.
The trial enrolled a total of 100 patients who were randomized into two groups. The experimental group received personalised treatment using the Reprieve System. The active comparator group received Optimal Diuretic Therapy.
The primary efficacy endpoint of the study is the total urine sodium output at 24 hours post-treatment initiation. The primary safety endpoint includes clinically significant acute kidney injury, symptomatic hypotension or hypertensive emergency. Secondary outcomes will assess the net fluid removal during treatment, heart failure rehospitalization and the total time on loop diuretics.
“Completing enrolment in the FASTR trial marks a significant milestone for Reprieve as we advance our mission to improve the lives of patients living with heart failure,” said Mark Pacyna, chief executive officer of Reprieve Cardiovascular. “We designed this study as a large, randomised pilot, which is uncommon in this space, to rigorously assess the safety and effectiveness of the Reprieve system in USA patients before embarking on a global pivotal trial. We would like to thank and recognise all the clinical research teams for their partnership in the FASTR trial. We look forward to advancing our clinical programme, with the initiation of a pivotal study before the end of the year and a data readout from the FASTR trial in the first half of 2025.”