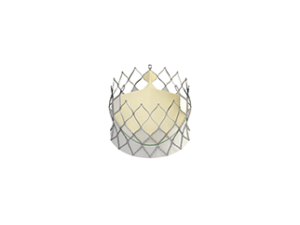
According to late-breaking trial presented at EuroPCR (16–19 May, Paris, France), a novel self-expanding transcatheter aortic valve implantation device (TAVI, Centera, Edward Lifesciences) is associated with excellent 30-day outcomes. A press release reports that the valve is associated with a high survival rate (99%) and a low rate of disabling stroke (2.5%) at 30 days. Furthermore, the valve was associated with a 4.9% rate of permanent pacemaker—the lowest rate ever reported in a multicentre trial for a self-expanding valve.
The rate of moderate paravalvular leak was also low (0.6%) and there were no incidences of severe paravalvular leak. All 203 patients in the study were treated via the transfemoral access route with the majority (174 patients) under conscious sedation.
The press release states that the Centera valve is repositionable and retrievable and can be delivered through a low-profile 14-French delivery system that features a motorized handle that results in stable valve deployment. It adds that the device is uniquely packaged—fully pre-attached that facilitates simple and rapid device preparation.
Study presenter Didier Tchétché (Clinique Pasteur in Toulouse, France) comments: “The Edwards Centera valve demonstrates extremely favourable early clinical safety and performance outcomes in the high surgical risk TAVI population. In addition to excellent patient outcomes, the valve also offers several unique features and an innovative tissue design, all of which simplify the procedure for clinicians.”
Centera-EU trial patients were enrolled in 23 centres in Europe, Australia and New Zealand and will be followed for five years. The valve is an investigational device not yet available commercially in any country. Edwards anticipates it will receive CE Mark approval for the device during the fourth quarter.










