 The European Investment Bank (EIB)—the investment arm of the European Union (EU)—is providing €20 million in venture-debt financing to German medical-technology company Protembis to develop a next-generation device for protecting the brains of patients who undergo certain heart treatments.
The European Investment Bank (EIB)—the investment arm of the European Union (EU)—is providing €20 million in venture-debt financing to German medical-technology company Protembis to develop a next-generation device for protecting the brains of patients who undergo certain heart treatments.
The funding is to support clinical trials, research, development and market access for the ProtEmbo cerebral embolic protection system.
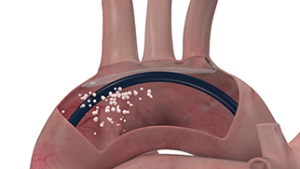
ProtEmbo is a filter device that deflects embolic material away from arteries to the brain during left-sided heart procedures including transcatheter aortic valve implantation (TAVI), countering risks including stroke and cognitive decline.
“This agreement demonstrates our commitment to supporting companies such as Protembis that aim to improve the health and wellbeing of European citizens. Their innovative technology, developed in Europe, will save patients who have to undergo heart surgery, from suffering grave side effects like cerebral embolic events”, said EIB vice-president Nicola Beer.
The EIB accord with Protembis is supported by the InvestEU programme to trigger more than €372 billion in additional investment in new technologies until 2027, a press release details. The deal is aligned with the InvestEU objective of promoting research, development and innovation.
“We are pleased to announce the signature of this agreement with the EIB,” said Protembis co-chief executive officers Karl von Mangoldt and Conrad Rasmus. “We would like to recognise the hard work, skill and professionalism of the EIB team while thanking our existing investors and our board for their unwavering belief in the benefits of this additional financing facility.”
ProtEmbo is inserted during TAVI via the artery in the left wrist, and lines the roof of the aortic arch, shielding the brain from the dislodged debris.
In March 2024, Protembis completed a €30 million series B financing round to advance a pivotal US Food and Drug Administration (FDA)-approved investigational device exemption study called the ProtEmbo trial. The trial will enrol between 250 and 500 patients undergoing TAVI in Europe and the USA. The study aims to show the superiority of ProtEmbo by randomising against a hybrid control group: half of this control-group receiving no protection and half receiving the Sentinel (Boston Scientific) system.











