 A pooled, individual patient-level data meta-analysis of two trials investigating the use of the Sentinel (Boston Scientific) cerebral embolic protection device has found no evidence of a reduction in stroke when the device is routinely used during transcatheter aortic valve implantation (TAVI).
A pooled, individual patient-level data meta-analysis of two trials investigating the use of the Sentinel (Boston Scientific) cerebral embolic protection device has found no evidence of a reduction in stroke when the device is routinely used during transcatheter aortic valve implantation (TAVI).
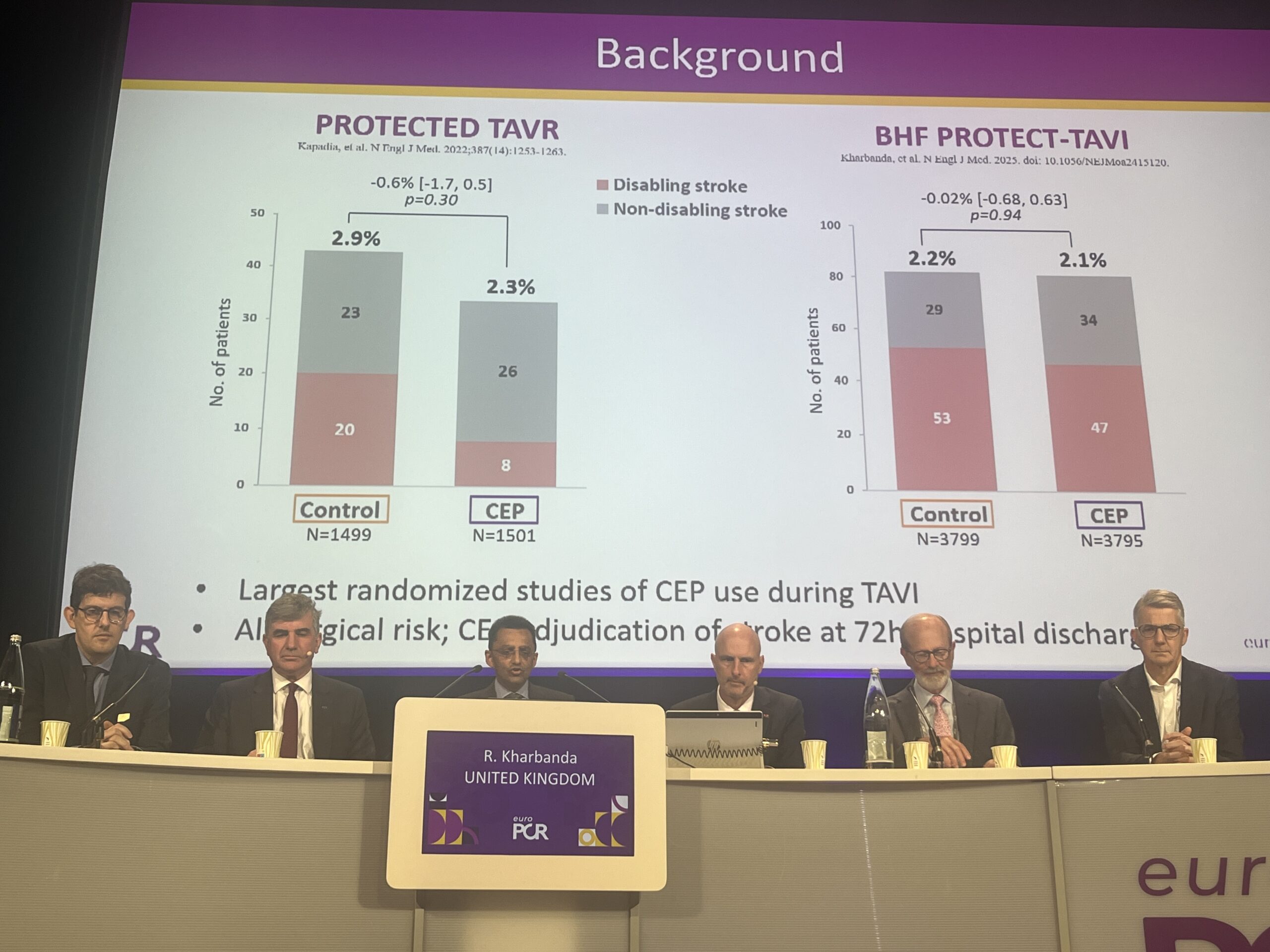
The analysis of the PROTECTED TAVR and BHF PROTECT TAVI trials, presented during a late-breaking trial session at EuroPCR 2025 (20–23 May, Paris, France), did show that severe, disabling strokes may be reduced among patients in whom the Sentinel device is fully deployed, according to investigator Rajesh Kharbanda (John Radcliffe Hospital, Oxford, UK), who revealed that work is ongoing to establish which are the patients at high risk for stroke who may confer a benefit from Sentinel.
“Stroke remains an issue even with modern day TAVI, and the Sentinel cerebral embolic protection device captures debris and reduces its incidence of going to the brain,” Kharbanda said in presenting the results of the analysis at a press conference at EuroPCR on Tuesday.
Taken together, PROTECTED TAVR, conducted in the USA and Europe, and BHF PROTECT TAVI, conducted in the UK, have enrolled more than 10,000 patients, with both trials neutral for the primary endpoint of all-cause stroke at 24 hours.
The latest study includes a primary endpoint of all-cause mortality with a secondary prespecified endpoint of disabling stroke, using a modified intention to treat approach. Investigators used a complier average causal effect (CACE) analysis to adjust for those patients whose devices were not deployed for the entirety of the procedure—for example due to complex anatomy caused by advanced peripheral vascular disease—and a specified per-protocol analysis of patients who received the device as intended with both filters successfully deployed.
In the modified intention to treat analysis, Kharbanda showed that the incidence of stroke stood at 2.3% in the group undergoing TAVI with Sentinel, versus 2.2% among those without, whilst in the per-protocol analysis of patients whose filters were present for the entire duration of the procedure these figures stood at 2.3% and 1.7% respectively. Using the CACE analysis, Kharbanda stated that there was no difference between the groups when adjusted for non-compliance. “The summary of that is that all-cause stroke is not reduced by this device,” he commented.
Looking at disabling stroke, the modified intention to treat analysis showed an incidence of 1.3% among patients undergoing TAVI with protection and 1% without, and 1.3% vs. 0.8% respectively within the per-protocol analysis. “The complier average causal effect analysis doesn’t show an effect on disabling stroke,” added Kharbanda.
“That is a complex set of data, and I want to summarise by saying that what this meta-analysis shows is that there is no evidence that, for all stroke, a routine strategy of embolic protection reduces incidence,” the presenter stated.
Any hint of an effect in a reduction in disabling stroke needs further analysis, Kharbanda stated, adding that the per-protocol analysis can be prone to post-randomisation error and selection bias.
“We need to understand what that signal means. As part of that, further work is under way to identify who is at risk of TAVI-associated stroke and therefore identify if cerebral embolic protection might be effective in that subgroup. Using this dataset, we will be able to answer some of those questions.”
Questioned by William Wijns (Lambe Institute for Translational Medicine, University of Galway, Galway, Ireland) on whether there were any suggestions as to which subgroups of patients may be the beneficiaries of the technology, Kharbanda said that it is “still early days” in looking at the data. However, he said that factors including a high surgical risk score, prior stroke and peripheral vascular disease may be at play. “I suspect it is not one factor, and that is the problem with univariate [analysis], when you look at single factors it is a combination of factors.”
Commenting whether the neutral findings of the trials have had any bearing on the usage of cerebral embolic protection, Kharbanda commented that there had been a downturn in the use of the Sentinel device corresponding with the release of the PROTECTED TAVR results. “When PROTECTED TAVR was published there was a small reduction in US use of the device, it wasn’t huge, but then it wasn’t widely used. There are still enthusiasts, and there are people who will say they have no problems with it, but the evidence supporting that is not really available yet.”










