Investigation of the use of an ultra-thin strut biopolymer sirolimus-eluting (BP-SES; Orsiro, Biotronik) compared to a durable polymer everolimus-eluting stent (DP-EES; Xience, Abbott), yielded no statistically significant difference in 30-day outcomes between the two devices, according to findings of the CASTLE study presented during a late-breaking trial sessions at EuroPCR 2021 (18–20 May, virtual).
The CASTLE study is an investigator-initiated, multicentre, single-blinded, randomised, non-inferiority clinical trial carried out in 69 centres in Japan, investigator Masato Nakamura (Toho University, Tokyo, Japan) told EuroPCR attendees during his presentation of the interim results of the study.
It has been anticipated that thinner stents have a potential to reduce vessel damage, inflammation, and thrombogenicity in patients undergoing percutaneous coronary intervention (PCI), Nakamura said, setting out the background to the study. Previous trials, he noted, have shown that ultra-thin strut sirolimus-eluting stents reduce target lesion failure (TLF), although the “true mechanism” of this superiority remains unclear.
Recent randomised trials have suggested that image-guided drug-eluting stent (DES) deployment reduces the risk of stent thrombosis and revascularisation rates, he added, noting that image-guided PCI is widely adopted in Japan. Therefore, Nakamura hypothesised that image-guided deployment may cancel the procedural factor and contribute to the clarification of the true effect of the ultra-thin stent.
The trial enrolled patients with acute and chronic coronary syndromes, who were randomised in a 1:1 ratio to image-guided PCI (intravascular ultrasound or optical coherence tomography) with BP-SES (intervention group) or DP-EES (control group).
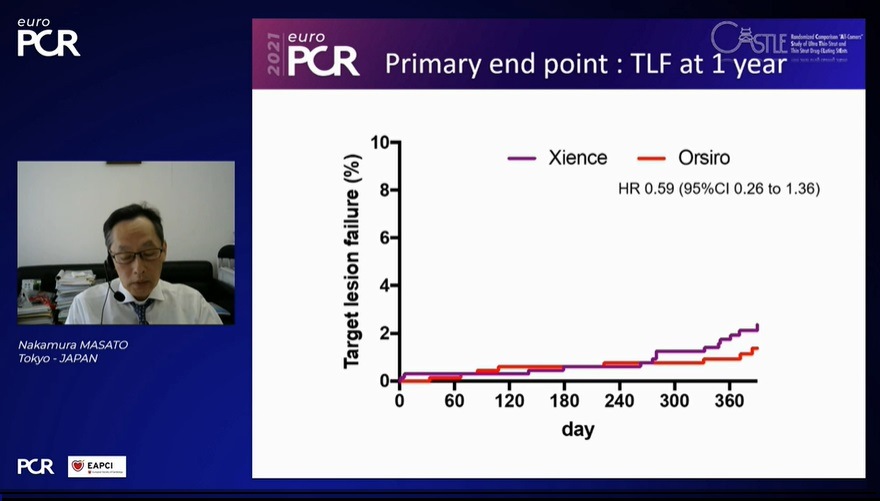
The primary outcome was target lesion failure (TLF), comprising cardiovascular death, target vessel myocardial infarction, and clinically driven target lesion revascularisation at a 12-month follow-up. An independent clinical event committee evaluated angiographies and clinical events. The prespecified margin for non-inferiority was 3.3%.
Nakamura reported that between May 2019 and March 2020, 1,440 patients were randomised; 722 were allocated to the BP-SES and 718 to the DP-EES. The 12-month follow-up was completed in 69.1% in the BP-SES group and 68.6% in the DP-EES group.
He relayed that there were no significant differences between groups in terms of clinical and procedural characteristics. The trial included mainly chronic coronary syndromes (85%), stent diameter ≤3mm (66%), and image-guidance was performed in at least 97.5% of the patients.
At 30-day follow-up, there was no difference in TLF between DP-SES vs DP-EES (5% vs 4.9%) or its components, Nakamura said. In the primary endpoint at the 12-month follow-up, there was no difference in TLF between BP-SES vs DP-EES (HR 0.59 [95%CI 0.26 to 1.36]).
According to Nakamura, the data in the interim analysis suggest that BP-SES and DP-EES may have similar clinical outcomes when PCI is performed under intracoronary imaging guidance. However, conclusions should not be drawn until the complete follow-up is performed to assess any potential difference between the two devices.












