Ancora Heart has announced that it has reached the first enrolment milestone in the CORCINCH-HF pivotal trial evaluating the AccuCinch transcatheter left ventricular restoration system in patients with heart failure with reduced ejection fraction (HFrEF).
Six-month follow-up data on these 250 patients will support the company’s premarket approval (PMA) submission to the US Food and Drug Administration (FDA).
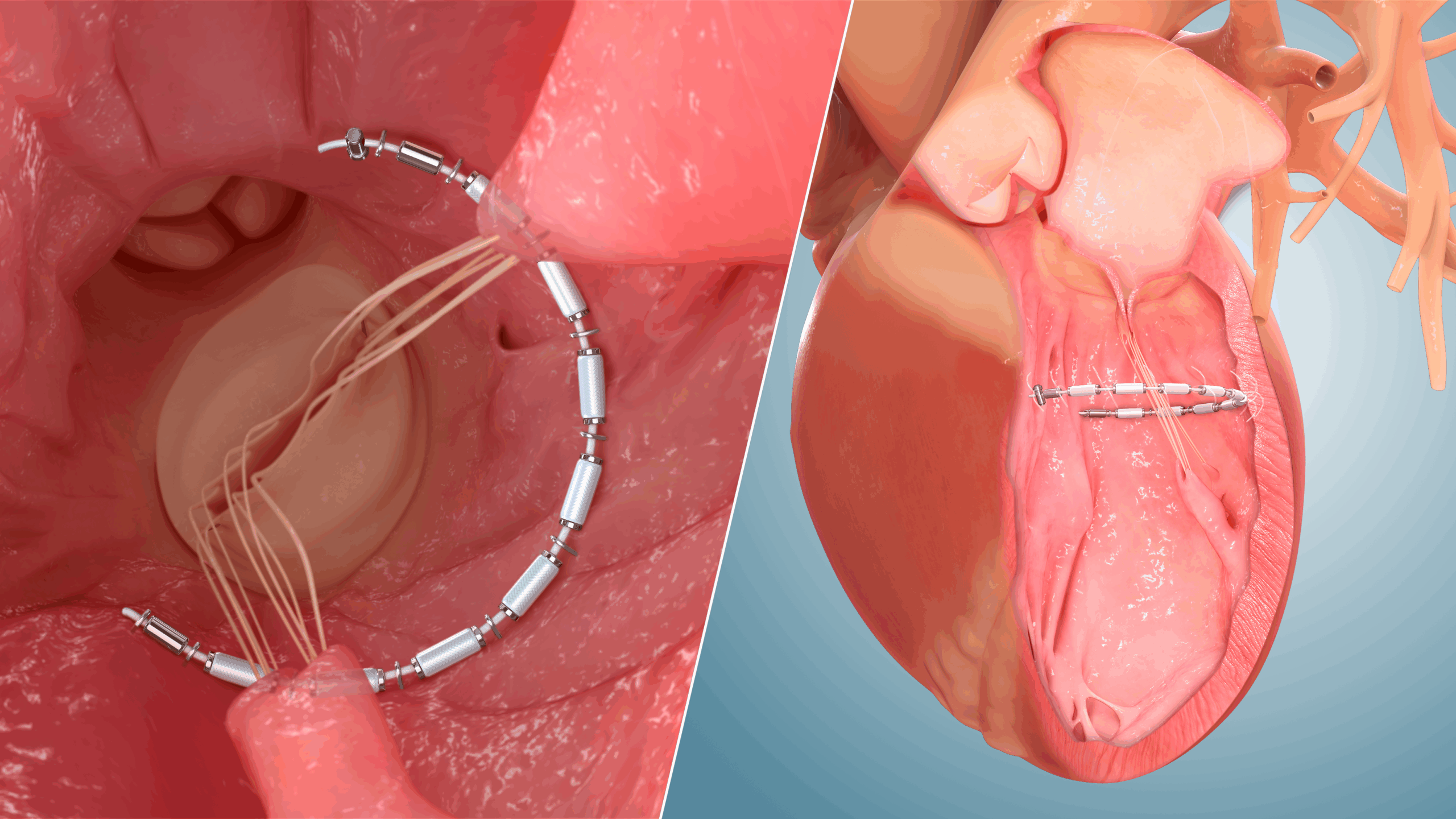
The AccuCinch system is an investigational device designed to reduce the size of the left ventricle, reduce ventricular wall stress, and support and strengthen the heart wall.
Results from early clinical studies were presented at the 2023 Technology and Heart Failure Therapeutics (THT) conference and simultaneously published in the Journal of Cardiac Failure. The AccuCinch system was granted breakthrough device designation from the FDA in 2022.

“The AccuCinch system is the only completely transcatheter procedure to treat the enlarged left ventricle,” said Jeff Closs, president and CEO of Ancora Heart. “Reaching this milestone is an incredible accomplishment in heart failure research and a model example of collaboration across heart failure and structural heart care teams at participating CORCINCH-HF clinical trial sites. We’d like to thank study investigators for their commitment to innovation and partnership, and we look forward to building on this momentum as we work toward reaching our full enrolment target of 400 patients.”
The CORCINCH-HF study is evaluating the safety and effectiveness of the AccuCinch system in patients who have symptomatic heart failure (HF) with reduced ejection fraction.
“Despite advances in guideline-directed medical therapy, many patients with heart failure continue to experience debilitating symptoms,” said Ulrich Jorde (Montefiore Health System, New York, USA), global co-principal investigator of the CORCINCH-HF study. “Reaching this milestone in the CORCINCH-HF study is a significant step toward determining whether this treatment option may improve the length and quality of their lives.”
“AccuCinch is a device-based therapy aimed at reverse remodelling of the enlarged left ventricle,” said Mark Reisman (NewYork-Presbyterian/Weill Cornell Medical Center, New York, USA). “This trial is designed to evaluate the safety of the device and procedure and whether we can improve heart structure and function and thereby help patients feel better, avoid hospitalisations and live longer.”










