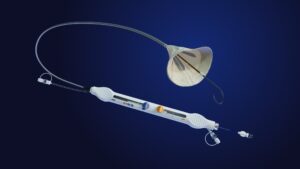
EMBLOK has announced that it has enrolled the first 50 patients in a clinical trial to evaluate the performance and treatment effect of its Emblok embolic protection system (EPS).
Explosive growth in transcatheter aortic valve implantation (TAVI) volumes has been reported globally, with some growth rates >50-fold according to a report from the American Heart Association (AHA). The TAVI market is estimated to increase at a compound Annual Growth Rate (CAGR) of 14.1% over the next decade in the USA alone.
The company’s press release states that TAVI offers a less invasive option for aortic valve replacement in the heart. During the replacement of the aortic valve, small pieces of calcium or tissue can sometimes break off from the old valve. This debris, referred to as emboli, can travel through blood vessels to the brain, placing a patient at risk for stroke. An embolic protection device captures emboli and mitigates the risk of emboli breaking free, protecting other vital organs during TAVI procedures. EMBLOK’s EPS device aims to offer whole-body protection from debris captured during TAVI, reduce procedural complexity and improve patient safety.
“The enrolment of the 50th patient in our study for our novel whole-body embolic protection system is a major milestone for the advancement of safety in TAVI procedures,” said Brad Brown, chief executive officer of EMBLOK. “Our mission is to provide a breakthrough solution that enhances patient safety during this increasingly prevalent procedure. The early results have been very encouraging, and we’re optimistic about the potential of this technology to set a new standard in embolic protection.”
The multicentre, single-blind, randomised controlled trial, Evaluation of safety and effectiveness of the EMBLOK EPS during TAVI, will enrol up to 532 subjects between 18–90 years of age with aortic valve stenosis who are undergoing TAVI. The company is initiating additional clinical sites and plans to finish enrolment by Q3 2025. Hemal Gada (UPMC Heart and Vascular Institute in Central, Pennsylvania, USA) and William Merhi (Corewell Health, Michigan, USA) will be co-principal investigators of the trial.
“Embolic protection is crucial during TAVI procedures to safeguard patients from the risk of stroke and other serious complications caused by debris dislodged during the procedure,” said Gada. “By ensuring comprehensive protection, we can significantly improve outcomes and enhance both the short and long-term quality of life for those undergoing this life-saving treatment.”
Cardiovascular pathologist, Renu Virmani (CVPath Institute, Gaithersburg, USA) commented: “As the field of transcatheter aortic valve replacement continues to evolve, the importance of whole-body embolic protection has never been more critical. While current systems target cerebral protection, capturing and removing debris from the entire circulatory system offers a more comprehensive safeguard. Whole-body embolic protection represents the next frontier in ensuring patient safety by minimising the risk of complications that can arise from debris dislodgement during TAVI procedures.”
“We’re thrilled to announce the formation of our advisory board, bringing together renowned experts who will help guide us as we continue advancing this important work and bring it to market,” continued Brown. “We see significant opportunities to, most importantly, support physicians performing TAVI procedures in their effort to optimise patient care, as well as play an integral role in the burgeoning TAVI market. These esteemed healthcare professionals will be instrumental to achieving our goals.”











