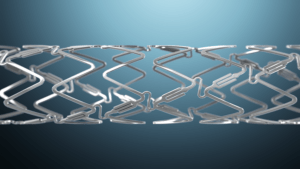
 Elixir Medical has today announced commencement of the INFINITY-SWEDEHEART randomised controlled trial (RCT) of the DynamX coronary bioadaptor system, which is described by the company as the first metallic coronary artery implant that adapts to vessel physiology.
Elixir Medical has today announced commencement of the INFINITY-SWEDEHEART randomised controlled trial (RCT) of the DynamX coronary bioadaptor system, which is described by the company as the first metallic coronary artery implant that adapts to vessel physiology.
The INFINITY-SWEDEHEART RCT is a prospective, multicentre, single-blind, randomised study enrolling 2,400 patients at approximately 20 sites in Sweden treated with the DynamX bioadaptor in a 1:1 randomisation to Resolute Onyx (Medtronic) drug-eluting stent (DES). The study will utilise the SWEDEHEART registry database to support patient follow-up.
The primary endpoint of the study is a device-oriented clinical endpoint (DOCE) of non-inferiority of target lesion failure (TLF), a composite endpoint that includes cardiovascular death, target vessel myocardial infarction, and ischemia-driven target lesion revascularisation, at one year. Powered secondary endpoints include superiority in the overall population and pre-specified sub-groups, including small vessel, long lesions, left anterior descending artery (LAD), chronic angina and diabetes.
David Erlinge, Lund University, Lund, Sweden, is the principal investigator of the INFINITY-SWEDEHEART RCT, and Stefan James, Uppsala University Hospital, Uppsala, Sweden, chairs the Executive Steering Committee for the trial.
The DynamX bioadaptor is a metal implant with a drug-eluting bioresorbable polymer coating that supports the coronary artery during healing, with radial strength similar to a DES. Over six months, the polymer coating dissolves, uncaging the bioadaptor and freeing the artery to move with the natural expansion and contraction of the artery, unlike DES. This has been shown to (a) maintain the ability for positive adaptive remodelling, (b) restore vessel function, and (c) allow for the vessel’s return toward baseline angulation.
The bioadaptor is designed to address the major adverse cardiac event (MACE) rate that occurs with drug-eluting stents each year without plateau. The rigid design of a DES constrains, or “cages,” natural artery movement, restricting its natural ability to accommodate disease progression. This has been associated with MACE.
Studies have shown adverse event rates associated with DES of 20% at five years and 40‒50% at 10 years. Published papers have demonstrated that a DES prevents positive adaptive remodeling, inhibits vessel compliance and dilation in response to the body’s changing blood flow needs, and causes vessel straightening, which has been associated with increased MACE.
“Drug-eluting stents have been very useful in opening blocked arteries, but the rigid stent structure cages the artery, impacting its ability to respond to disease progression and maintain blood flow lumen, as it would otherwise do naturally,” said Erlinge. “We are enthusiastic about studying the novel DynamX device, which is the first metallic device treating coronary artery disease that has been shown to restore pulsatility and positive adaptive remodelling of coronary arteries. The benefits of restored vessel function may provide greater benefit in patients with complex disease. The INFINITY-SWEDEHEART study includes these complex patients with both Chronic Coronary Syndrome and Acute Coronary Syndrome.”
“Swedish quality registries, including SWEDEHEART, have been utilised by Uppsala Clinical research center (UCR) for a number of groundbreaking cardiovascular clinical trials and it is appropriate that a unique device such as the DynamX device would be evaluated in a large number of patients using its infrastructure,” said James. “We have found the device as easy to deliver as a stent, but with the potential to reduce long-term DES-associated risks and improve upon patient outcomes over time.”
“The INFINITY-SWEDEHEART RCT utilising the respected SWEDEHEART registry database is an efficient approach to conducting an evidence-based trial, and provides important benefits. For the first time, we will be able to compare the performance of the physiologically-adaptive DynamX against an industry-leading DES in the overall study population and in subsets of patients. In addition, by leveraging the existing rigorous SWEDEHEART registry database, we will be able to conduct the trial and follow-up with high quality, efficiency and speed,” said Motasim Sirhan, Elixir Medical’s CEO.