Patients awaiting cardiac surgery who underwent a digital “prehabilitation” programme benefited from an improvement in major adverse cardiovascular events (MACE) compared to patients who did not undergo the same intervention prior to their surgery.
This is the conclusion of the Digital Cardiac Counseling Trial, a randomised trial led by investigators in Maastricht, The Netherlands, who—during the COVID-19 pandemic—used a digital application to deliver patients a series of rehabilitation modules, tailored to their specific modifiable risk-factors, aimed at improving outcomes following their procedure.
Results of the trial were presented at the European Association of Cardio-Thoracic Surgery (EACTS) 2024 annual meeting (9–12 October, Lisbon, Portugal), and simultaneously published in the Journal of the American College of Cardiology (JACC), in what has been described as a first for research presented at a cardiothoracic surgery meeting.
“Cardiac surgery saves lives, there is no doubt, but what if we could improve patient outcomes by starting rehabilitation programmes weeks before the upcoming surgery?” posed study investigator Bart Scheenstra (Maastricht University Medical Center, Maastricht, The Netherlands) in his presentation of the results at the EACTS meeting.
“We already know from previous research in cardiac prehabilitation that it improves quality of life, it reduces length of hospital stay, and it reduces complications. We do not know what the effect is of these programmes on major adverse cardiovascular events,” he said.
To investigate this, the trial included 394 patients scheduled for elective cardiac surgery or transcatheter interventions who were referred at several centres throughout The Netherlands to the Maastricht University Medical Center. Patients enrolled in the trial were either randomised to the investigational group (n=197), where they were given access to an online multimodal teleprehabilitation programme, or assigned to a control arm (n=197), where no additional intervention was given.
Patients were screened prior to randomisation, and those in the intervention group were offered remote rehabilitation modules that included a smoking-cessation programme, nutritional counselling, psychological-education, inspiratory muscle training, and exercise training, based upon their individual risk factors, delivered over a period of around six to eight weeks.
Scheenstra said that the trial cohort resembled a “typical outpatient surgery population”, with an average age of 66 years old, 75% male, and with a Euroscore of 1.1. Around 40% were scheduled for coronary surgery, 40% for valve surgery, and 20% for other types of cardiac surgery.
The MACE endpoint consisted of a composite of cardiovascular death, myocardial infarction (MI), stroke, hospitalisation for heart failure or other life-threatening cardiac events, and earlier or repeated intervention. Outcomes were then assessed by an independent, blinded, events adjudication committee.
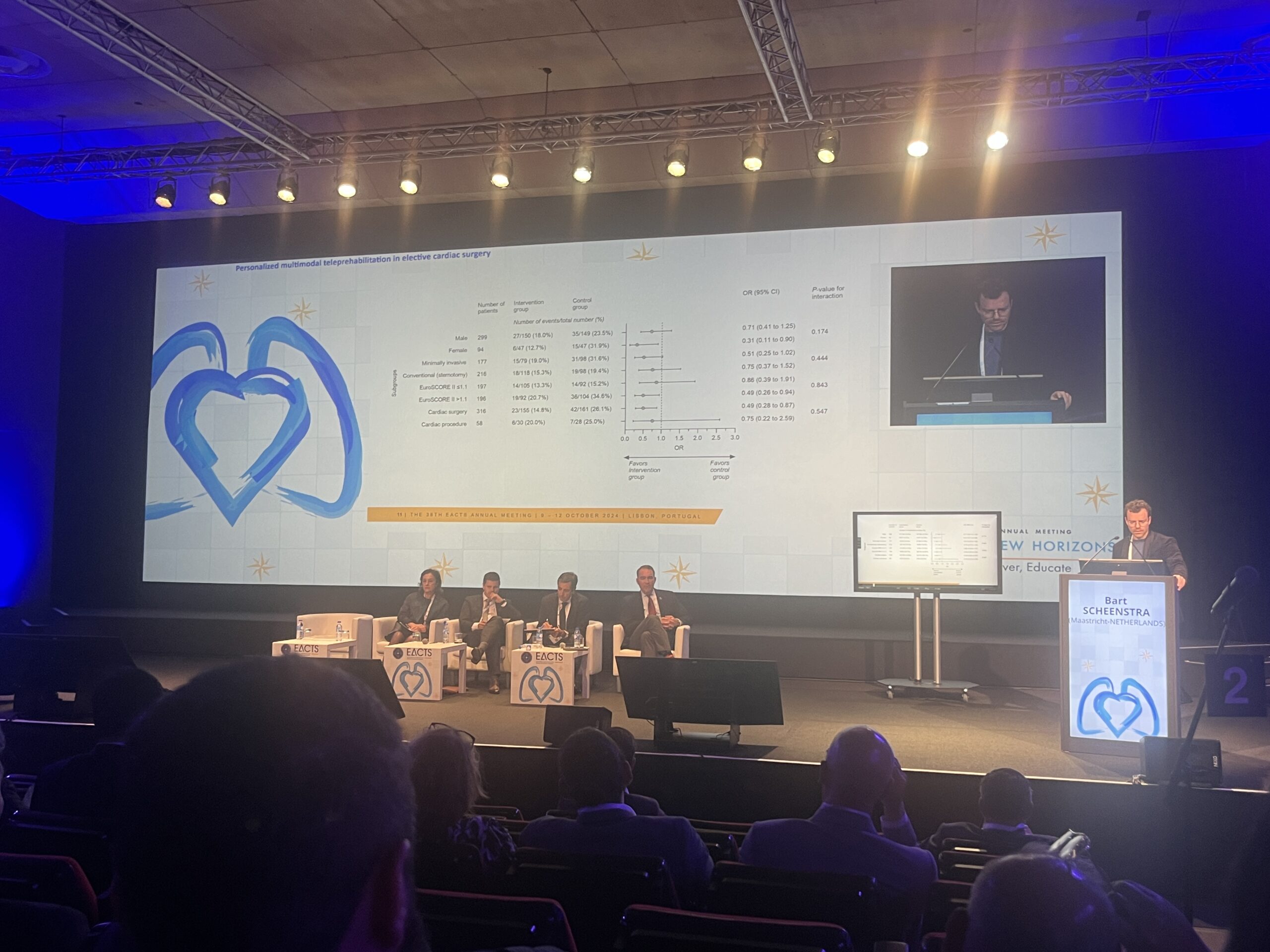
Results of the trial presented by Scheenstra and detailed in JACC showed that from randomisation until one year postoperatively, the primary endpoint occurred in 33 patients (16.8%) in the teleprehabilitation group and 50 patients (25.5%) in the control group.
The difference was primarily driven by a reduction in hospitalisations, with sensitivity analyses showed that treatment effect was mainly in the patients undergoing cardiac surgery rather than transcatheter procedure.
Teleprehabilitation also reduced the incidence of active smokers, elevated pulmonary risk scores, and elevated depression scores. There was no significant difference in postoperative length of hospital stay, occurrence of postoperative complications, physical fitness, incidence of obesity, or malnutrition the study shows.
“What is really important here is that this is a trial that empowered patients to take care of their care, and to do something about their risk factors at their own home,” the trial’s principal investigator Peyman Sardari Nia (Maastricht University Medical Center, Maastricht, The Netherlands) commented at the EACTS meeting. “Even if they don’t participate in all those modules for which they actually have risk factors, this trial shows that you will still have an effect, because it is possible to integrate into the care that we already have, because it moves the care to the patients’ home.”