Results of a trial involving cell therapy in patients with chronic heart failure due to low ejection fraction (EF) have been published in the Journal of the American College of Cardiology (JACC).
DREAM-HF—Double-blind randomized assessment of clinical events with allogeneic mesenchymal precursor cells in heart failure—which was sponsored by Mesoblast, was a phase 3 trial performed at 51 sites in 565 patients with chronic heart failure, who were also on standard-of-care heart failure treatment.
The study, which had a mean follow-up of 30 months, was designed to examine the effects of mesenchymal precursor cells (MPC) on the number of hospitalisations and major adverse cardiovascular events in heart failure.
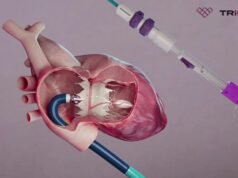
MPCs are seen as a candidate for use in heart failure with low EF as they have the potential to improve myocardial perfusion and contractility while reversing cardiac and systemic endothelial dysfunction, the study’s authors, Emerson C Perin (The Texas Heart Institute, Houston, USA) et al note. The cells were obtained from the bone marrow of healthy adult donors. Cell-treated patients in the study received direct cardiac injections of MPCs, and control patients underwent a “sham” or mock procedure with no injections.
Though the primary (time-to-recurrent events caused by decompensated heart failure with reduced ejection fraction) and secondary endpoints (time-to-first terminal cardiac events and all-cause death) of the trial were negative, investigators reported that the therapy benefited patients by improving the heart’s pumping ability, as measured by EF, and reducing the risk of heart attack or stroke, especially in patients who have high levels of inflammation. They also note that a strong signal was found in the reduction of cardiovascular death in patients treated with cells.
Furthermore, MPC-treated patients showed significant strengthening of the left ventricular muscle within the first 12-months as measured by an increase in left ventricular EF, and over a mean follow-up of 30-months, treatment with MPCs reduced the risk of cardiovascular death, heart attack, or stroke, with a greater decrease in patients with increased inflammation.
MPC treatment reduced the rate of heart attack or stroke by 58%, and the benefit rose to 75% in patients who had high levels of a blood marker for inflammation.
MPC therapy did not further reduce recurrent heart failure events requiring hospitalisation over and above the effects of traditional drugs which reduce circulating volume overload caused by the maladaptive effects of neurohormonal activation.
“The results of DREAM-HF are an important step in understanding how cell therapy provides benefits in patients with chronic heart failure due to poor pump function,” said Perin. “The cells appear to work by reducing inflammation, increasing microvascular flow, and strengthening heart muscle.
“Locally, in the heart, the MPCs can protect cardiac muscle cells from dying and can improve blood flow and energetics. In large blood vessels throughout the body, the reduced inflammation resulting from the activation of MPCs may decrease plaque instability, which is what leads to heart attacks and strokes. The cells seem to have a systemic immune modulatory and anti-inflammatory effect.”