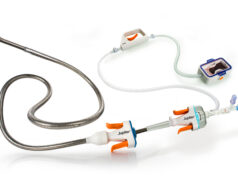
Abbott has received US Food and Drug Administration (FDA) clearance and CE mark for its Amplatzer Piccolo delivery system, which is used with the company’s Amplatzer Piccolo occluder.
Abbott has received US Food and Drug Administration (FDA) clearance and CE mark for its Amplatzer Piccolo delivery system, which is used with the company’s Amplatzer Piccolo occluder.
The new delivery system is designed specifically to treat premature babies (some weighing as little as two pounds) with a hole in the heart known as a patent ductus arteriosus (PDA).
“Abbott’s new Amplatzer Piccolo delivery system is a transformative step forward in how we treat PDA in premature infants,” said Evan Zahn (Cedars-Sinai Medical Center, Los Angeles, USA). “The new delivery system simplifies the implant procedure because only one catheter is needed instead of multiple, and a shorter and softer design allows for more precise device positioning in these tiny babies. Doctors can treat this group with more confidence, reducing the risk of adverse events and improving the long-term outlook for this uniquely vulnerable patient population.”
The Amplatzer Piccolo device is inserted through a small incision in the infant’s leg and guided through vessels to the heart using the Amplatzer Piccolo delivery system, where it is placed to seal the opening in the heart. The Amplatzer Piccolo Occluder received FDA approval and CE Mark in 2019.
“We designed the Amplatzer Piccolo delivery system based on feedback from leading physicians across the world to make PDA closure procedures even safer and easier,” said Sandra Lesenfants, senior vice president of Abbott’s structural heart business. “With the Amplatzer Piccolo Occluder, which is the world’s smallest heart device, and now with the new delivery system to complement it, we’re continuing to advance how we meet the needs of our tiniest patients with structural heart disease.”