DeepQure, a Seoul based medical device company with a novel, extravascular solution for renal denervation, has announced initiation of its early feasibility study (EFS) for the HyperQure system following US Food and Drug Administration (FDA) investigational device exemption (IDE) approval.
The company describes HyperQure as the world’s first extravascular renal denervation device for the treatment of resistant hypertension.
With this approval, the company will commence the early feasibility study (EFS) to prove the safety and efficacy of HyperQure in 15 patients with resistant hypertension. The clinical trial will be conducted in a prospective, multicentre, single-arm, open-label design at major US university hospitals, including Stanford University, Mayo Clinic, Emory University, University of Arizona and the University of California, Irvine.
“We are thrilled that the FDA has approved our IDE study plan. This is a significant US regulatory milestone for DeepQure, starting the feasibility study using the extravascular ablation platform in the USA for the renal denervation indication. We will accelerate our global clinical trials with this IDE approval,” says Chang Wook Jeong, co-founder and chief medical officer of DeepQure.
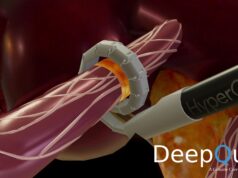
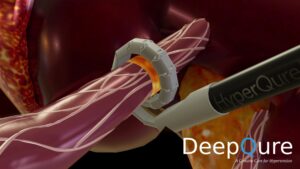
HyperQure system consists of an energy generator and a laparoscopic instrument that delivers radiofrequency (RF) energy for ablation directly to the sympathetic nerves around the renal artery by wrapping the renal artery 360 degrees from outside of the vessel.
This mechanism allows full denervation of renal sympathetic nerves without damaging the vascular endothelium which has proven to be a challenge for intravascular systems.