Pending the results of several trials and “iterative changes” to device design, bioabsorbable scaffolds are set to change the treatment paradigm for lower extremity peripheral arterial disease (PAD) within the next decade. So concluded Eric Secemsky (Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, USA) during a presentation at the Leipzig Interventional Course (LINC 2025; 28–30 January, Leipzig, Germany).
Secemsky was speaking on the future of bioabsorbable scaffolds, providing an overview of the key features of this “ideal” device type for the treatment of below-the-knee (BTK) disease. The first characteristic is drug elution, he noted, which inhibits restenosis and provides drug-modulated healing for sustained patency; second, a scaffold design provides transient support in the vessel and addresses recoil and dissection; and finally, resorbability ensures nothing is left behind.
“The era of bioabsorbable scaffolds has really begun with the Esprit drug-eluting resorbable scaffold (DRS),” Secemsky commented, noting that this Abbott innovation “is what created the market in the USA”. Esprit DRS is the first device of its kind to receive approval from the US Food and Drug Administration (FDA), the presenter shared, highlighting positive data from the prospective, multicentre, randomised LIFE-BTK trial that now has results available out to two years.
Secemsky stressed, however, that the technology is still in its infancy. The Esprit BTK device is “only the first step on a long pathway to solving BTK interventions for both US and non-US patients,” he told the LINC audience.
Here the presenter listed a number of other devices with bioabsorbable properties that are being evaluated in the USA, focusing in particular on the Motiv (Reva Medical) and Magnitude (R3 Vascular) scaffolds.
The Motiv device, Secemsky detailed, is CE mark approved, has been granted FDA breakthrough designation, and is currently being assessed in the recently completed MOTIV BTK randomised controlled trial. Led by Ehrin Armstrong (Adventist Heart and Vascular Institute, St Helena, USA) and Andrej Schmidt (University of Leipzig, Leipzig, Germany), the trial enrolled its target cohort of 292 patients across 35 centres in the USA and Europe and is awaiting completion of follow-up.
“We saw results of their early postmarket trial in the EU looking at 60 limbs, 58 patients, and [the device] demonstrated really impressive patency outcomes through three years,” Secemsky shared. Continuing, the presenter stressed the significance of this result: “It’s hard for a BTK device in vessels that are less than 3.5mm to maintain 88% patency through 36 months.”
Moving on to Magnitude, Secemsky disclosed that he is the co-principal investigator for the US pivotal trial of this device. “[Magnitude] is really as resistant to compression and supportive as a metallic stent, but has unique flexibility to optimally perform in challenging anatomical locations,” Secemsky noted, also pointing out the device’s ability to “maintain its structure in a compromised situation, including in the infrapopliteal space”.
The results of early feasibility study RESOLV I, Secemsky shared, were “very supportive of moving forward to an IDE [investigational device exemption] trial”. In total, he noted that 36 lesions in 35 limbs were treated in this trial with >90% freedom from occlusion or restenosis, which included “really impressive real-world patien t and lesion characteristics”.
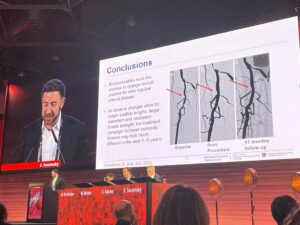
“Bioabsorbables have the promise to change clinical practice for infrainguinal arterial disease,” Secemsky posited, drawing his presentation to a close.
The presenter continued, considering timelines: “As iterative changes allow for longer scaffold lengths, larger diameters and consistent tensile strength, the treatment paradigm for lower extremity disease may look much different in the next five to 10 years.
“I think we’ll continue to have these conversations over the years, see more data, and I think we’ll see many of these scaffolds make it to clinical practice.”










