Observational data from Sweden’s national registry on coronary and valvular interventions—SWEDEHEART—have shown no difference in rates of mortality, heart failure hospitalisation and stroke among patients receiving the Acurate neo2 (Boston Scientific) when compared to Sapien 3 (Edwards Lifesciences) and Evolut (Medtronic) transcatheter aortic valve implantation (TAVI) systems.
The results of the analysis, presented during a late-breaking trial session at the 2025 Cardiovascular Research Technologies (CRT) meeting (8–11 March, Washington DC, USA), offer some reassurance over the performance of the Acurate neo2 platform, after the device failed to meet non-inferiority for the same endpoints in the ACURATE IDE trial, a prospective, multicentre, randomised study, intended to support regulatory approval for the Acurate platform in the USA, which is already widely used outside of the country.
Andreas Rück (Karolinska University Hospital Stockholm, Stockholm, Sweden) presented the analysis of SWEDEHEART data, explaining that the address—a “target trial emulation study” —sought to address some of the concerns thrown up by the results of the ACURATE IDE trial.
Results of ACURATE IDE, reported at the 2024 TCT meeting (27–30 October, Washington, DC, USA), showed that one-year composite rates of all-cause mortality, stroke or rehospitalisation stood at 16.16% among patients receiving Acurate neo2 compared to 9.53% in the control arm with the comparator device, meaning that Acurate neo2 did not meet the prespecified 8% margin for non-inferiority.
Investigators looked at possible factors contributing to the finding, discovering that as many as 21% of Acurate neo2 valves implanted were under-expanded.
“This result was shocking, but at least led to the fact that we, as big users of Acurate, needed to reassure ourselves that this valve is safe to use,” explained Rück of the rationale for this latest analysis.

Using the observational data from SWEDEHEART, Rück and colleagues emulated all aspects of the ACURATE IDE trial protocol including eligibility criteria, follow-up time and outcomes. They found 1,943 TAVI patients who fit the criteria for the study, 644 receiving Acurate neo2 and 1,299 receiving either Evolut or Sapien 3 valves.
The population was described as typical for those seen in European TAVI practice, with a mean age of 81.2 years and 49.8% female. Most patients (99.8%) receiving Acurate neo2 underwent predilatation, whilst post-dilatation was also frequent, occurring in 44.7% of cases compared to just 26.1% of cases in the IDE trial.
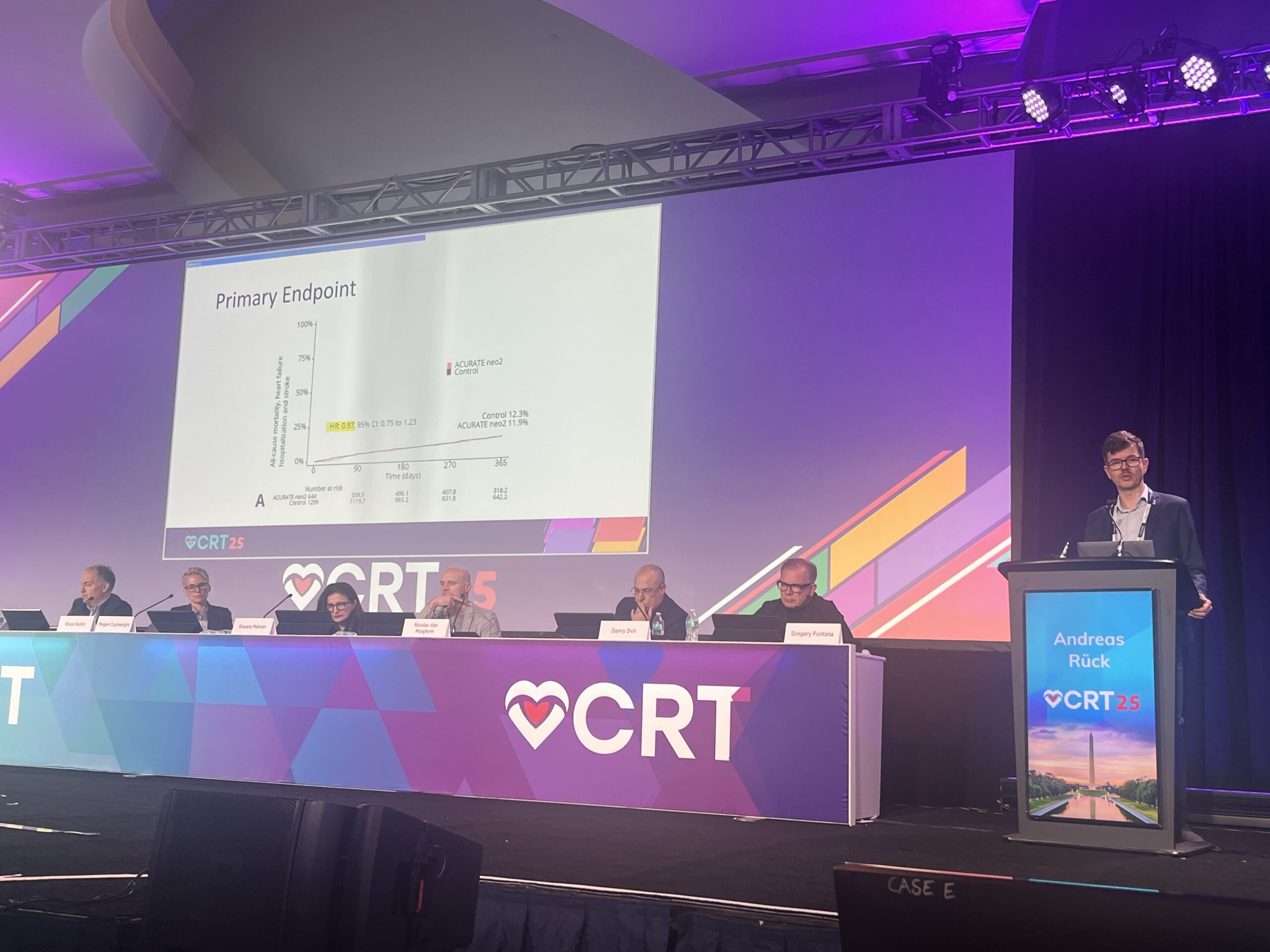
Rück reported that the primary endpoint of all-cause mortality, heart failure hospitalisation and stroke occurred in 11.9% of patients receiving Acurate neo2 compared to 12.3% in the control group. Event rates for each of the components of the composite endpoint were virtually identical, with all-cause mortality trending lower the Acurate neo 2 valve.
Outlining key differences in the procedures within the Swedish study and the ACURATE IDE trial among patients receiving the Acurate neo2 valve, Rück noted that predliatation was done in almost all cases in both trials, though there was a big difference in balloon sizing between the two datasets, with very few balloons within a 1mm annular diameter range in the IDE trial. Additionally, Rück reported a substantial difference in the post-dilatation balloon sizing, with 74% within 1mm of the annular range in the Swedish data compared to 49% in the IDE trial.
“If we try to compare our data from Sweden with the IDE trial, we see that our patients were a little bit older, and the severity of the aortic stenosis was actually a little bit higher, both of those would lead to more events potentially,” Rück summarised. “Predilatation was done almost in 100% in both, but the balloon sizing was a big difference in the IDE trial, with very few balloons within 1mm. Post-dilatation was less frequent in the IDE and there was also a big difference in the sizing.
“At the end of the day, the postoperative mean gradient was very similar between these two, and interestingly the rate of new pacemakers at 30 days was actually lower in the Swedish data.”
The presenter noted that the findings were limited by the observational data, and it was not possible to ascertain how many of the valves were fluoroscopically under-expanded—a key take-away from the IDE trial.
Analysis of the IDE trial data, which were shared at TCT 2024 suggested that under-expansion of the valves was a key contributor to the trial’s negative result.
“Under-expanded valves had higher flows, turbulent flow and reduced washout. That is a very plausible reason for platelet activation and clumping possible micro-emboli which could affect later strokes and later myocardial ischaemia. When we look at time to event, what we saw with under-expanded valves was that you had double the risk of all-cause mortality and triple the risk of stroke,” ACURATE IDE trial investigator Michael Reardon (Houston Methodist DeBakey Heart & Vascular Center, Houston, USA) commented upon presentation of the results.
Commenting on the results of the SWEDEHEART data at CRT 2025, Nicolas van Mieghem (Thoraxcenter, Erasmus University Medical Center, Rotterdam, The Netherlands) noted the significance of the differences in the handling of the valve in each of the trials.
“There are a lot of learnings and takeaways from this study. The main one is how we handle the Acurate valve in Europe is definitely different to how the valve was handled in the IDE trial.
“You demonstrated the importance of balloon dilatation pre and post, and the rule of thumb these days is that when you do a balloon dilatation for the Acurate valve it is a 1:1 sizing, for pre- and post-dilatation. That means that you just rely on one balloon for both, that is a very important from this trial and the IDE trial.”
Speakers at CRT were asked if these latest data could help support a regulatory submission for Acurate neo2 to the US Food and Drug Administration (FDA), in lieu of positive data from the IDE trial.
“There’s no question that it is encouraging to see that the hypothesis that under-dilatation was the issue and you show us that with diligent work that could be fixed,” said Roxana Mehran (Mount Sinai Hospital, New York, USA) on this point. “Am I convinced in a non-randomised study 100% that I feel like this is just as good? It is a difficult one to sell, because it is observational, but I think it is great to see that and hopefully in a smaller sized [trial] in a randomised fashion, I am certain there are ways that we could do this so that they could continue to be looked at.”

Commenting on the data presented by Rück at CRT, Reardon described the findings as “reassuring”.
“In the IDE study we failed to meet our non-inferiority endpoint. This led us to do a post hoc analysis where we found almost a quarter of the Accurate valves were under-expanded. Fully expanded valves had no difference in mortality or stroke at one year compared to the control valves. Under-expanded valves had an increased risk of both death and stroke at one year compared to control.
“The IDE trial was enrolled during the Covid-19 pandemic waves and took 47 months to enrol. The average time between Accurate neo2 implantation at sites was 3.9 months.
“In the SWEDEHEART study, operators were implanting the Acurate neo2 valve as part of their normal TAVI program. The SWEDEHEART implanters also followed the guidance for pre-implantation balloon dilatation sizing much more commonly than what was done in the IDE study.
“We have now clearly defined for US operators how to recognise and treat under-expansion. The hope, of course, is that this will lead to results seen in the expanded group of the IDE which matched control valves. With the excellent results seen in Europe, we are hopeful to find a path forward in the USA.”












The SWEDEHEART data offers valuable insights into the performance of the Acurate neo2 valve in real-world settings, contrasting sharply with the concerning results of the ACURATE IDE trial. It’s fascinating to see how procedural differences, particularly in balloon sizing and pre/post-dilatation practices, appear to play a critical role in outcomes. This highlights a key question: are device shortcomings to blame, or do we need more rigorous training and standardization in TAVI protocols? If the latter, it underscores the importance of bridging gaps between clinical trial conditions and real-world practices. Could this ultimately redefine how we evaluate and approve devices for broader markets like the U.S.?