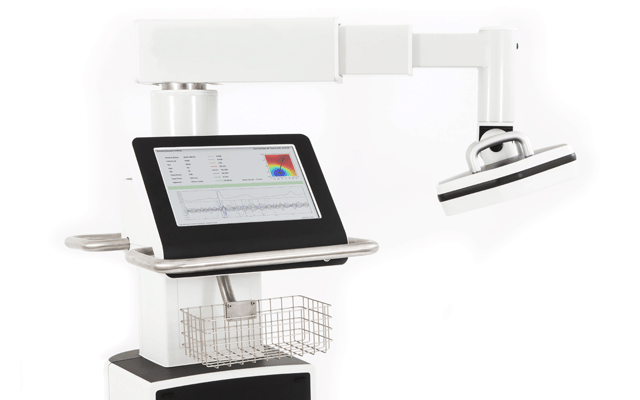
The US FDA has granted 510(k) clearance to Creavo Medical Technologies for its portable scanning device, Vitalscan. A press release reports that the move opens the US market for the device and demonstrates an increasing industry focus on the global challenges hospitals face when it comes to triaging chest pain patients.
The press release adds that clinical trials will now start in a number of centres across the USA in addition to the multicentre trial already taking place at four major UK emergency departments.
Vitalscan uses magnetocardiography (MCG) and is designed to enable a quick, non-invasive three to five-minute scan at a patient’s bedside. The aim of the scan is to help physicians accurately rule-out ischaemic heart disease, meaning that patients can then go on to the most appropriate care pathway for their needs rather than go through the current lengthy and resource intensive process.
Steve Parker, CEO, Creavo Medical Technologies says: “This is a major development and brings us closer to realising our overriding objective – to improve patient care and save time and resources for hospitals. Engagement with the FDA represents yet another significant achievement in what has been a very successful period for us. In the past year we have received CE mark registration in Europe and our large-scale, multi-centre UK clinical trial is continuing to progress well. We also raised a further US$17m in an oversubscribed funding round, enabling us to continue to build momentum and extend our reach further. We know that the technology has the potential to be a game changer and our ability to go from strength to strength is further evidence of this.”








